Chemistry-Atomic structure & the periodic table
- Created by: Maria is the best
- Created on: 17-12-17 13:35
Structure of the atom
The structure of an atom is shown by nuclear model =atoms have small nucleus orbited by electrons - nucleus is positive as contains postively charged protons and neutral neutrons(size of nucleus=1/10,000 size of atom) - electrons that orbit nucleus are negatively charged meaning atoms don't have an overall charge - electrons occupy shells around nucleus - is very small
Elements
Element=made up of 1 type of atom. The ma** no.=total proton+neutrons in nucleus. Atomic no.=no. of proton/electrons. To find out neutron no. -> ma** no. - proton no.
In every element is equal no. of positive and negative electrons.
Electronic configuration : way to expre** how many electrons in each shell (e.g a=2,8,8)
Atomic Mass + Molecular Mass
Ar = Relative Atomic Mass (no. of protons + neutrons in atom/mass no.)
Mr = Molecular Mass (sum of relative atomic mass in each element in compound/molecule)
e.g --> Ar of Fe (Iron) = 56 Mr of CO2 (carbon dioxide) = 12 + (16x2) = 44
Isotopes
Atoms of same element always of same no. of protons BUT no. of neutrons can change.
Isotopes are different forms of same element, which have same no. of protons but different no. of neutrons = have same atomic no. but different mass no.
How to find relative atomic mass of isotope=
How much of an element is represented in compound (%) formula =
Ar of element required Mr
100
Paper Chromatography
Chromatography used to separate mixtures made up of liquids of different colours. An e.g. of this is the use of paper chromatography to separate different dyes in an ink. Here are the steps:
- draw a line near bottom of a sheet of filter paper (use pencil as pencil marks insoluble and won't dissolve in solvent)
- put spot of ink on line (small amount), use different known dyes to compare and see what ink made of. Then put sheet upright in beaker of solvent. Solvent depends on what's being tested. some compounds dissolve well in water but some don't and other solvents (ethanol) needed. ink shouldn't touch solvent initially - don't want it to be washed away
- Place lid on top of container to stop solvent evaporating
- solvent will seep/travel up paper, carrying ink with it
- different dyes in ink will move up at different rates=dyes will separate out+form dots in different places. If any dyes in ink insoluble in solvent=will stay on baseline
- the point the solvent has reached as it moves up paper known as solvent front. when solvent front nearly reached top=take paper out of beaker, draw line with pencil along solvent front + leave to dry
- end result=pattern of spots=chromatogram
Paper Chromatography Diagram
Analysing Paper Chromatography
Are different types of chromatography but all have 2 phases: Mobile phase - where molecules can move (liquid/gas) and Stationary phase - where molecules can't move (solid/thick liquid).
During experiment, substances in sample constantly move between two phases - equilibrium formed. Mobile moves through stationary&anything dissolved in mobile moves with it. How quickly chemical moves depends on how 'distributed' between 2 phases - whether spends more time in mobile or stationary.
Theory of paper chromatography - during it stationary phase=filter paper + mobile=solvent. The amount of time molecules spend in each phase depends on: how soluble they are & how attracted they are to paper. Molecules with higher solubility in solvent & which are less attracted to paper=more time in mobile=carried further up paper
Analysing Paper Chromatography...
Result of chromatography experiment=chromatogram. Each substance on chromatogram has Rf value=ratio between distance travelled by dissolved substance (from baseline to centre of spot) & distance travelled by solvent.
The further through the stationary phase the substance moves=larger Rf value. Can calculate Rf value using formula:
More Separating Techniques
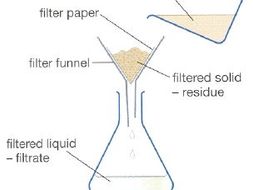
Separating insoluble solid + liquid - Filtration often used if desired product=insoluble solid that needs to be separated from liquid reaction mixture=also useful technique for purification.
For e.g., solid impurities in reaction mixture can be removed using filtration. Here's how its done:
- fold a piece of filter paper into cone. Can do this by folding paper in 1/2 and then again, once opened get cone shape
- place filter paper point down into filter funnel that's sitting in neck of container (conical flask)
- pour mixture containing insoluble solid into funnel lined with filter paper. Make sure none of mixture goes over top/down side of filter paper
- liquid=pass through filter paper but solid won't = left behind in funnel
Filtration Diagram
Separating a soluble solid and a solution
If solid can be dissolved=soluble. Are 2 methods used to remove soluble product from solution - evaporation & crystallisation.
Evaporation = quick way of separating soluble salt from solution but can only use if salt doesn't decompose when is heated otherwise have to use crystallisation. Method:
- pour solution into evaporating dish
- place evaporating dish on top of tripod&gauze and place a Bunsen burner underneath
- slowly heat solution. solvent will evaporate and solution=more concentrated. eventually solid will start to form
- keep heating evaporating dish until all you have=dry solid
Evaporation Diagram
Crystallisation
Crystallisation=takes more time than evaporation, however can produce nice crystals=would decompose if heated. Method:
- place evaporating dish on top of tripod with gauze mat. Place bunsen burner underneath tripod
- pour solution into evaporating dish & gently heat it. some of solvent=evaporate and solution=more concentrated
- once some of solvent evaporated/when see crystals start to form (point of crystallisation)=remove dish from heat + leave solution to cool
- salt should start to form crystals as becomes insoluble in cold, highly concentrated solution
- filter crystals out of solution&leave in warm place to dry. Could also use drying oven or desiccator (sealable enclosures)
Crystallisation Diagram

Distillation
Simple Distillation = used to separate liquid from mixture. used in industry to get pure water from sea water. Method:
- equipment set up in diagram and mixture heated. component of mixture that has lowest boiling point evaporates
- as vapour rises passes into condenser where is cooled and condenses and is collected in container below condenser
- components of mixture with higher boiling points left behind in flask
Simple distillation=separating substances using boiling points significantly apart from each other. But if boiling points of substance close to each other=doesn't work. temp my rise above boiling point of more than 1 of substances & end up mixing again.
Simple Distillation Diagram
Fractional Distillation
Fractional distillation=used for separating mixture of different liquids + esp. useful when b.p of liquids close together. commonly used in industry (crude oil) also used in lab. Method:
- using equipment set up. place mixture in flask, attach fractioning column on top+heat it
- different liquids=different b.p=evaporate at different temps
- liquid with lowest b.p evaporates 1st. when temp on thermometer matches b.p of the liquid, vapour=reached top of column&passed into condenser. will then cool and condense and run out of end. pure liquid can then be collected
- liquids with higher b.p may also start to evaporate.but colum cooler towards top=only get part of way up before condensing&running back down towards flask
- when 1st liquid collected, you raise temp to next lowest b.p of liquids in mixture...
Fractional Distillation Diagram

The History of the Atom
The ideas&theories of atoms started by ancient greeks=word 'atom' comes from ancient greek 'atomos'-meaning indivisible. Means ancient greeks thought all matter could be divided into smaller units until 'indivisible' particle=atom. But wasn't evidence=not much progress until English Chemist John Dalton described atoms as 'solid spheres' + said made up different elements.
However in 1897 JJ Thompson=shown from experiments that atoms not 'solid spheres' or 'indivisible' but with measurements of charge+mass=showed atom held even smaller, negatively charged particles=electrons->plum pudding model made. Model=atomic structure suggested by JJ Thompson=reveals atom as sphere of positive charge with negatively charged electrons in it.
Plum Pudding Model

History of Atom...
In order to prove plum pudding model correct in 1909 Ernest Rutherford + assistants conducted alpha particle scattering experiment. What happened: a beam of alpha particles aimed at extremely thin sheet of gold. As plum model stated scientist=expecting alpha particles to pass straight through as positive charge of each atom thought to be spread out atom. Some alpha particles had gone straight through BUT some=went through foil at different angles+some even came back. This experiment disproved plum pudding model.
Rutherford Experiment Diagram
Rutherford Experiment Diagram 2
History of Atom...(cont.)
Rutherford then used evidence from his experiment + showed atom must contain positively charged central nucleus=called nuclear atom model.
However, Niels Bohr further developed Rutherford's model. Bohr's model of atom proposed that electrons=shells and not floating randomly. Bohr thought electrons orbited nucleus in shells not anywhere in between. Each shell fixed distance from nucleus. Bohr's theory=supported by many experiments+helped discovery of protons&neutrons later.

Related discussions on The Student Room
- Mass vs relative atomic mass on periodic table »
- Chemistry relative atomic mass »
- chemistry question »
- Dissociation chemistry »
- Chemistry help »
- Chemistry question Orbitals »
- Four atoms of an unknown element weigh 5.24718 »
- Relative atomic mass »
- Chemical structures Biology A level »
- Shapes of molecules »

Comments
No comments have yet been made