cells and microscopes
- Created by: lucie reynolds
- Created on: 24-05-21 17:31
cells and microscopes
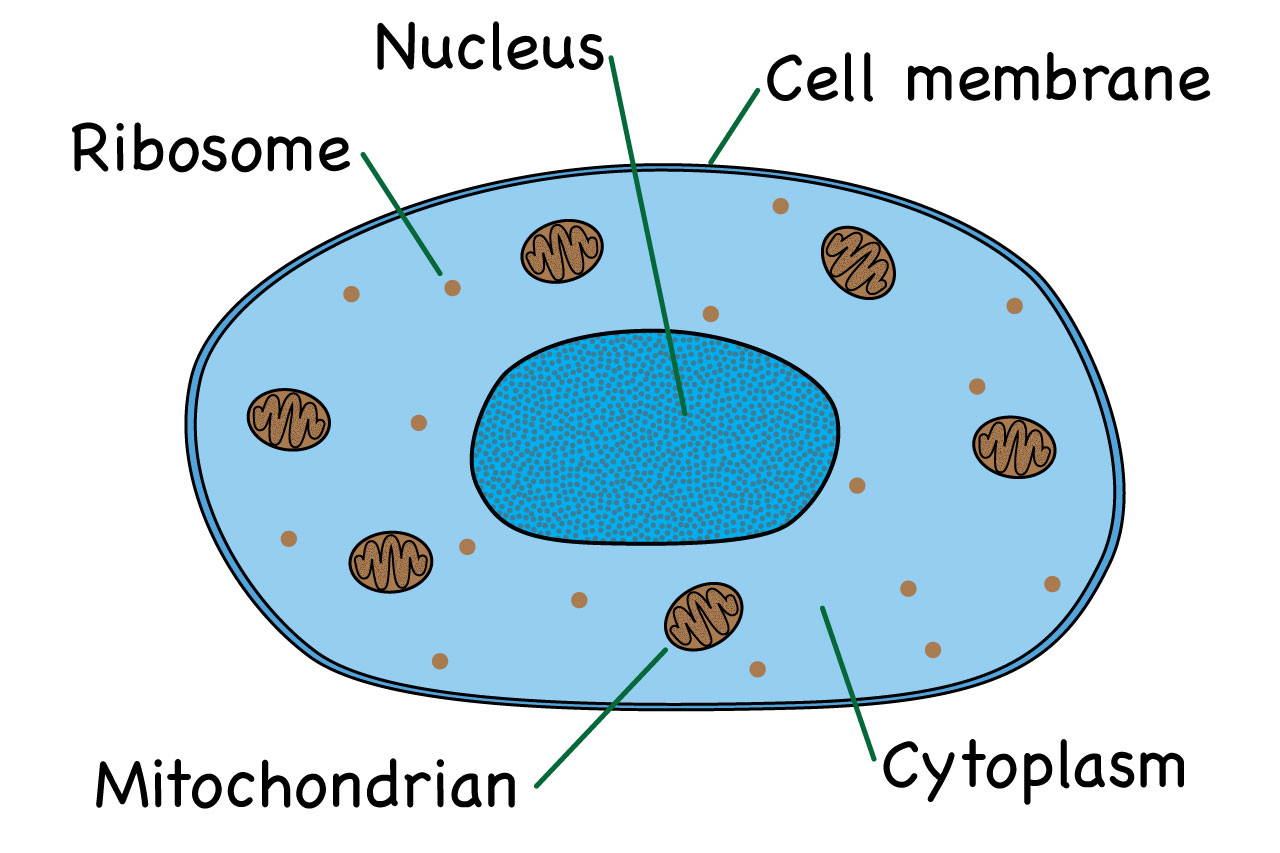 cytoplasm- site of chemical reactions in the cell. gel like subsatance containing enzymes to catalyse reaction.
cytoplasm- site of chemical reactions in the cell. gel like subsatance containing enzymes to catalyse reaction.
nucleus- conatins genetic material, controls the activietes of the cell and codes for proteins.
cell membrane- semi-permeable. controls the movemnet of subsatnces in and out of the cell.
ribosome- site of protein synthesis. proteins are built from amino acids.
mitrochondira- site of respiration. where energy is released for the cell to function
magnification (M)= size of image(I) / real size of object(A)
diffusion, osmosis and active transport
Diffusiuon- movement of particles in a solution or gas from a higher to lower concentration. eg. O2 and CO2 in gas exchange. factors that affect the rate are surface area and temperature.no energy required.
osmosis- movement of water from a dilute solutuion to a more concentrated solution. eg. plants absorb water through osmosis through their root hair cells. no energy required.
active transport- movement of particles from a dilute solution to a more concentrated one. eg. movement of mineral ions into roots of plants. energy is required.
factors affecting the rate of reaction
factors affecting rates of reaction
temperature- the higher the temperature, the quicker the rate of reaction.
concentration- the higher the concentration, the quicker the rate of reaction.
surface area- the larger the surface area of a reactant solid, the quicker the rate of reaction.
pressure(of gases)- when gases react, the higher the pressure upon them, the quicker the rate of reation.
activation energy, collision theory and catalysts
collison theory- Chemical reactions can only occur when reacting particles collide with each other with sufficient energy.
activation enrgy- This is the minimum amount of energy colliding particles in a reaction need in order to react.
Increasing the temperature= increases the frequency of collisions, makes the collisions more energetic,increasing the rate of reaction.
Increasing the concentration, pressure (gases) and surface area (solids) of reactions=increases the frequency of collisions, increasing the rate of reaction.
catalyst -A catalyst changes the rate of a chemical reaction but is not used in the reaction.
enzymes-These are biological catalysts.
how do they work?-Catalysts provide a different reaction pathway where reactants do not require as much energy to react when they collide.
rate of chemical reaction
rate off chemical reaction-
This can be calculated by measuring the quantity of reactant used or product formed in a given time.
Rate = quantity of reactant used
time taken
Rate = quantity of product formed
time taken
atoms, elements, compounds and mixtures
atom- the smallest part of an elemnt that can exist-have a radius of around 0.1NM and have no charge (0).
element- conatins only one type of atom.- around 100 differnet elemts and each one can be represented by a different symbol.
compound- two or more elements chemically bonded-compounds can only be separated into elements by chemical reactions.
mixtures- two or more elements ore compounds not chemically bonded together- csn be separated by physical proscesses.
developement of the model of the atom
pre 1900- tiny solid spheres that can not be divided.(john dalton)
1897-plum pudding- a ball of postive charge with negative electrons embedded in it.( jj thompson)
1909-nuclear model- postively charged nucleus at the centre surrounded by negative electrons (ernest rutherford)
1913-bohr model- electrons orbit the nucleus at specific distances (neils bohr)
james chadwick- provided the evidence to show the existence of neutrons within the nucleus
separating mixtures
filtration- separating an insouluble solid from a liquid. eg. to get sand from a mixture of sand, salt and water
crystallisation-to separate a solid form a solution. eg. to obtain pure crystals of sodium chloride from salt water.
simple distillation- to separate a solvent form a solution. eg. to get pure water from salt water
chromotography- separating subsatances that move at differnt rates through a medium. eg. to separate out the dyes in food colouring.
relative charges of subatomic partlicles
mass number-(top number) the sum of the protons and neutrons in the nucleus
atomuic number-(bottom number) the number of protons in the atom
number of protons=number of electrons.
Name of Particle
Relative Charge
Relative Mass
Proton
+1
1
Neutron
0
1
Electron
-1
Very small
halogens
With metals
Forms a metal halide
Metal + halogen à metal halide
e.g. Sodium + chlorine à sodium chloride
With hydrogen
Forms a hydrogen halide
Hydrogen + halogen à hydrogen halide
e.g. Hydrogen + bromine à hydrogen bromide
With aqueous solution of a halide salt
A more reactive halogen will displace the less reactive halogen from the salt
Chlorine + potassium bromide à potassium chloride + bromine
halogens 2
Halogens
Consist of molecules made of a pair of atoms
Have seven electrons in their outer shell. Form -1 ions.
Melting and boiling points increase down the group (gas à liquid à solid)
Increasing atomic mass number.
Reactivity decreases down the group
Increasing proton number means an electron is more easily gained
group 1 metals
Alkali metals
Very reactive with oxygen, water and chlorine
Only have one electron in their outer shell. Form +1 ions.
Reactivity increases down the group
Negative outer electron is further away from the positive nucleus so is more easily lost.
group 1 metals 2
With oxygen
Forms a metal oxide
Metal + oxygen à metal oxide
e.g. 4Na + O2à 2Na2O
With water
Forms a metal hydroxide and hydrogen
Metal + water à metal hydroxide + hydrogen
e.g. 2Na + 2H2O à 2NaOH + H2
With chlorine
Forms a metal chloride
Metal + chlorine à metal chloride
e.g. 2Na + Cl2à 2NaCl
metals and non metals and group 0
Metals
To the left of the Periodic table
Form positive ions. Conductors, high melting and boiling points, ductile, malleable.
Non metals
To the right of the Periodic table
Form negative ions. Insulators, low melting and boiling points.
Noble gases
Unreactive, do not form molecules
This is due to having full outer shells of electrons.
Boiling points increase down the group
Increasing atomic number.
energy 1
energy stores(J)- chemical energy, kinetic energy, gravitational potential energy, elastic potential energy.
chemical energy(J)- transferred during chemical reactions eg. fuels,foods or battereis
kinetic energy(J)-all moving objects have it 0,5 x mass x (spped)^2
1/2 x m x v^2
gravtiational potential enrgy(J)- stored in an object lifted up. GPE= massx g x height
Ep= m x g x h
elastic potential energy(J)= energy stored in a springy object
energy 2
energy can be transferred by- heatting(therman enrgy always flows from hot to cold objects), an electrical currunt flowing, a force moving an object
useful energy(J)- energy transferred to the place and in the form we need.
wasted energy(J)- not useful. eventualy transferrred to surroundings.
work done(J)- equal to the energy transferred, when a force moves an object
work done= force x distance moved W= f x s
conservation of energy- energy can not be created or destroyed only transferred,stored or dissapated.
hooke's law and the K sping constant- the extension of a sping is proportional to the force on it.
power- energy(J) transfreed in 1 second power(W)=energy(J) / time(S)
energy 3
wasted power- total power in - useful power out
radioactivity 1
radioactive decay- unstable nuclei emitting a type of radiation
random event- you can not predict or change when decay might happen
alpha particle- two neutrons and two protons. stopedby paper or skin. range a couple of cm in air highly ionising:has charge of +2 parent atom drops by 4 and atomic no.drops by 2
beta particle- a high speed electron made when a neutron turns into a proton. stopped by thin aliminium. range up to 1m. mid ionising: has charge of -1. parnet atom remain the samee and the atomic number rises by 1
gamma ray- an electromagnetic wave. stopped by thick lead. unlimited range. low ionising. parent and atomic no. remain the same.
activity- rate of unstavle nuclei decay measured in Bq
irradiated- exposed to radiation but does not become radioactive.
radioactivity 2
radioactive contamination - unwanted presence of radioactive material.
geiger counter- nuclear radiation detector
half-life- time it takes for ther radiactive nuclei to halve or the time it takes for the activity to halve.
isotope- same number of protons but different number of neutrons
chadwick- discovered neutrons
Related discussions on The Student Room
- Do all light microscpes only view living cells? »
- Biology »
- Exam help gcse bio edexcel »
- mark scheme biology 2022 gcse combined science higher paper 1 »
- Test revision notes gcse edexcel higher biology »
- Anyone done the Edexcel Biology B A level Core practical 2 - Use a light microscope.. »
- AQA GCSE Biology Paper 1 (Higher Tier) 8461/1H - 17 May 2022 [Unofficial Mark Scheme] »
- AQA A Level bio Cells »
- homework help »
- AQA A Level Chemistry Cells »
Comments
No comments have yet been made