C2
- Created by: Sophie_Stevens00
- Created on: 28-01-16 17:49
Ionic Compounds 2
- However when have been melted the ions are free to move.
- This allows them to carry an electrical charge.
- Some ionice substance dissolve in water because the water molecules can split up the lattice.
- So the liquids conduct electricity.
- The ions are free to move in the solutions and so they also conduct electricity.
The strong forces of attraction between the oppositly charged ions make the compound very strong.
Covalent bonding 1
- A covalent bond is a strong bond between non-metal atoms. It consists of a shared pair of electrons. It can be represented by a straight line or dot and cross diagram.
- The number of colvalent bonds an atom forms depends on the number of electrons it needs to achieve a stable electron arrangement.
- Hydrogen and chlorine can each form one covalent bond, oxygen two bonds, nitrogen three, carbon four bonds.
Convalently bonded substances fall into two main types:
- Simple molecules
- Giant covalent structures
Ionic Compounds 1
- Ionic bonding occurs between positive and negative ions, which attract each other and bind together to form ionic compounds.
- Ionic compounds have giant structures in which many strong electrostatic forces hold the ions together.
- They are solids at room temp.
- Lots of energy is needed to overcome the ionic bonds to melt the solids.
- Therefore ionic compounds have high melting and boiling points.
Ionic bonding - dot and cross diagrams 5
Sodium Cloride - Example
The electron on the highest enegry level of the sodium atom is lost and bonded to the clorine atom, which has gained the sodium electron, to make a full outer shell. Now both of the atoms have a full outer shell. This is ionic bonding.
Ionic bonding 3
- Ionic bonding is the transfer of charged electrons to form ions.
- Metal atoms lose the electron(s) in their highest energy level and become positively charged ions.
- Non-metal atoms gain electron(s) from another atom to become negatively charged ions.
Ionic bonding 4
Magnesium (Mg) - Example
Magnesuim is in Group 2. It has two electrons in its highest energy level. When these electrons are lost, a magnesuim ion Mg2+ is formed.
Giant Covalent Structures 3
Many convalently bonded substances consists of small molecules. Some atoms that can have serval bonds, like carbon, can join together in giant covalent structures.
- Diamond (a form of carbon) - Each carbon atom is joined to four other carbon atoms, forming a giant covalent structure. As a result, diamond is very hard and has a high melting point. It does not conduct electricity.
- Graphite (a form of carbon) - A substance in which the atoms join in flat 2D layers. There are weak forces between the layers and so they can slide over each other, making it much softer than diamond. Each carbon atom in a layer is joined to only three other carbon atoms, so graphite has delocialised electrons along its layers and so its conducts electricity.
- Silica - contains silicon and oxygen atomd, instead of carbon atoms. It is also hard and has a high melting point. The fact that it is a semi-conductor makes it immensely useful in the electronics industry: most transistors are made of silica.
Simple molecules - Intermolecular forces
Inter = between and molecular = molecules.
- The forces of attraction between the molecules, called 'intermolcular forces', are very weak.
- Hydrogen, ammonia, methane and water are simple molecules with covalent bonds. All have very strong bonds between the atoms, but much weaker forces holding the molecules together. When one of these substances melts or boils, it is these weak 'intermolecular forces' that break, not the strong covalent bonds. This means the substance made of small molescules have low melting and boiling points.
Covalent bonding - Dot and Cross Diagrams 2
A molecule of water
These conatin only a few atoms held together by strong covalent bonds. an example is water (H2O), the molecules of which cotain one atom of oxygen with two atoms of hydrogen.
- Oxygen has 6 electrons on its highest energy levels so it needs 2 more electrons to fill the outer shell. Hydrogen only has 1 electron, so to form water you must convalently bond 2 hydrogen atoms, sharing the 2 electrons, making the covalent bond H2O.
Nanoscience and Nanotechnology
- Nanoscience is about structures that are a few nanometres in size.
- A nanometre is one billioth of a metre.
- They contain a few hundered atoms arranged in a particular way.
- Their stuctures and very small sizes give them new properties that can make them very useful materials.
Properties and uses of nanoparticles
Working with nanoparticles is called nanotechnology.
Nanoparticles have very large surface areas, exposing many more atoms at their surface then normal materials, so they are often able to react very quickly. This make them useful as catalysts to speed up reactions. They can, for example, be used in self-cleaning ovens and windows.
Nanoparticles can be used...
- Self-cleaning ovens and windows.
- Cosmetics such as sun screens to block harmful ultraviolet light without appearing white on the skin and also deodrants
- Highly efficient catalysts
- New coatings and constuctions materials with special properties
- Make drugs more effective
Giant covalent structures 4
The structers of graphite, diamond and silica - From left to right.
Simple molecules 5
Properties of simple molecular substances
- Low melting and boiling points - This is because the forces between simple molecules are weak, so many of the substances are gases or liquids at room temperture.
- REMEMBER - O2, H2 and Cl2 are all gases at room temperture and these are simple molecules
- Non-conductive - They don not conduct electricity. This is because they do not have any free electrons and have no overall charge.
3.1 Mass numbers
- The mass number of an atom is the total number of protrons and neutrons in its nucleus.
- protron + neutrons = mass number
- The atomic number is the number of protrons it contains
- Protrons and neutrons have an equal mass
- relative mass of 1 unit
Isotopes
- Isotopes are atoms of an element with the normal number of protons and electrons, but different numbers of neutrons. Isotopes have the same atomic number, but different mass numbers.
- The different isotopes of an element have identical chemical properties. However, some isotopes are radioactive.
Isotopes of chlorine:
chlorine atoms contain 17 protrons and 17 electrons. About 75 % of chlorine atoms have 18 neutrons, while about 25% have 20 neutrons.
17 protrons, 17 electrons, neutons = 35 - 17 = 18
17 protrons, 17 electrons, neutrons = 37 - 17 = 20
Reversible reactions
Reversiable reaction go in both directions. The products can react to produce the original reactants again (go back to its original substance).
Represented with the symbol:
Reversiable reactions are exothermic in one direction and endothermic in the other direction.
Example of a reversible reaction
- Anhydrous copper(II) sulfate + water
hydrated copper(II) sulfate
- The reaction between anhydrous copper(II) sulfate and water is used as a test for water. The white solid turns blue in the presence of water.
When heating blue copper sulfate crystals, the water that was present has now evaporated into the surroundings and the colour of the sulfate has changed to a white powder, this is anhydrous copper sulfate. When added water, the reaction has reversed back to its orginal substance, hydrated copper sulfate.
Chromatography
Chromatography can be used to separate mixtures of coloured compounds. Mixtures that are suitable for separation by chromatography include inks, dyes and colouring agents in food.
- A spot of the mixture is placed near the bottom of a piece of chromatography paper and the paper is then placed upright in a suitable solvent, eg water.
- As the solvent soaks up the paper, it carries the mixtures with it.
- Different components of the mixture will move at different rates.
- This separates the mixture out.
Further Chromatography
To save time in analysising chromatograpy , chemists use the retention factor (Rf).
This identifies the different chromatograms and the separated components of the mixtures by using the equation:
Rf = distance moved by the compound ÷ distance moved by the solvent
Rates of reaction
The rate of a reaction measures the speed of a reaction or how fast it is.
There are two ways to measure the rate of a reaction:
- Measure the rate at which a reactant is used up
- Measure the rate at which a product is formed
For example, if 24 cm3 of hydrogen gas is produced in two minutes, the rate of reaction = 24 ÷ 2 = 12 cm3 hydrogen / min.
The faster the rate, the shorter the time it takes for the reaction. So the rate is inversely porportional to time.
Collision theory
- The collision theory states that reactions can only happen if particles collide.
- The collision must have enough energy for the particles to react.
- The minimum energy needed for particles to react is called the ACTIVATION ENERGY
- The collision must have enough energy for the particles to react.
Factors that affect the chance of collisions, increasing the rate of reaction:
- Temperture
- Concentartion of solutions
- Using a catalysts
- Surface area of solids
- Pressure of gasses
The effect of temperature
Increasing the temperture increases the rate of reactions
If the temperature is increased:
- The reactant particles move more quickly
- More particles have the greater activation energy
- The particles collide more often, and more of the collisions result in a reaction
- The rate of reaction increases
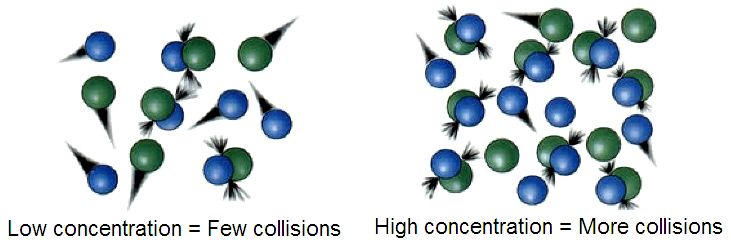
The effect of concentration
Increasing the concentraction of reactants, or the pressure of a gas increases the rate of reactions.
If the concentration of a solution is increased, or the pressure of a reacting gas is increased:
- There are more reactant particles in the same volume
- There is a greater chance of the particles colliding
- The rate of reaction increases
Equal volumes of gases at the same temperature and pressure contain equal number of molecules (particles).
The effect of catalysts
Catalysts increase the rate of chemical reactions without being used up.
They do this by lowering the activation energy needed of a reaction so that more collisions results in a reaction, incresing the rate of reaction.
Industry:
- They are expensive however they do not need replacing very often.
- They reduce energy costs and time needed for reactions.
- Different reactions need different catalysts.
Exothermic reactions
Exothermic reactions transfer energy to the surroundings, heating it up and so the temperture increases.
The energy is usually transferred as heat energy, causing the reaction mixture and its surroundings to become hotter. The temperature increase can be detected using a thermometer.
Some examples of exothermic reactions are:
- Burning fuels and metals (combustion)
- Neutralisation reactions between acids and alkalis
- The reaction between water and calcium oxide
REMEMBER - Reversiable rections are exothermic in one direction and endthermic in the other direction.
Endothermic reactions
Endothermic reactions take in energy from the surrondings, causing the reaction mixture and its surroundings to decreas in temperature.
The temperature decrease can also be detected using a thermometer.
Some examples of endothermic reactions are:
- Electrolysis
- The reaction between ethanoic acid and sodium carbonate
- The thermal decomposition of calcium carbonate in a blast furnace
- Photosynesis uses light energy in an endothermic reaction.
Electrolysis
- Electrolysis is the process by which ionic substances are decomposed (broken down) into elements when an electric current is passed through them.
- It requires a liquid to coduct the electrixcity, called the electrolyts.
- Electoloytes cotain free ions - they're usually the molton or dissiolved ionic substance.
- Its the free ions which conduct the electricty and allow the whole process to work.
- For and electrical circuit to be complete, there's got to be a flow of electrons.
- Positively charged ions move to the negative electrode during electrolysis. They receive electrons and are reduced.
- Negatively charged ions move to the positive electrode during electrolysis. They lose electrons and are oxidised.
- Metals or hydrogen are formed at the negative electrode.
- Non-metallic elements are formed at the positive electrode.
Electrolysis - Example
Lead bromide
When we pass electricity through moltn lead bromide it forms molten lead and brown bromine gas, as the electrolyte is broken down by electricity.
lead bromide contains positively charged lead ions and negatively charged bromide ions.
Reduction and Oxidation
- Positively charged ions gain electrons at the negatie electrode
- Negatively charged ions lose electrons at the positve electrode
OILRIG
Oxidation Reduction
Is Is
Loss of eletrons Gain of electrons
Acids and alkalis
The pH scale
The chemical properties of many solutions enable them to be divided into three categories - acids, alkalis and neutral solutions. The strength of the acidity or alkalinity is expressed by the pH scale.
The universal indicator changes colour depending on whether it's above or below a ceratin pH. It's vey useful for esimating the Ph of a solution
Acids and bases
Bases are substances that can react with acids and neutralise them. Bases such as metal oxides and metal hydroxides react with acids to form neutral products.
Examples of bases include:
- copper(II) oxide
- zinc hydroxide
An alkali is a soluble base, a base that can dissolve in water.
Examples of alkalis include:
- sodium hydroxide
- potassium hydroxide.
All alkalis are bases
Neutralisation
When an alkali is added to an acid the pH of the mixture rises. This is because the alkali reacts with the acid to form neutral products (or vise versa). This is neutralisation
Related discussions on The Student Room
- Maths for economists - constrained maximisation questoin »
- circle question help »
- Learning a 2nd language »
- Why study modern languages instead of study abroad? »
- Isaac physics SPC 2022 »
- Edexcel GCSE Geography B Paper 2 (1GB0 02) - 9th June 2023 [Exam Chat] »
- factors effecting bond strength »
- Senior Physics Challenge Isaac Physics »
- How do I derive the order of growth in this code? »
- Energy dissipated »
Comments
No comments have yet been made