Chemistry Unit 1
0.0 / 5
- Created by: YOIMO
- Created on: 16-05-17 19:30
C1.1.1 Atomic Structure
- Elements are arranged in order of their atomic numbers in the periodic table
1 of 23
C1.2.1 Limestone And Its Uses
- We get limestone by quarrying
- Blocks of limestone are used for buildings
- Limestone is used to make calcium oxide and cement
- limestone is mainly calcium carbonate CaCO3
- When heated strongly, calcium carbonate decomposes to make calcium oxide and carbon dioxide. This is done on a large scale in lime kilns.
- Equation: CaCO3 -> CaO + CO2 This is thermal decomposition- breaking down, by heating
2 of 23
C1.2.2 Reactions Of Carbonates
- All metal carbonates react in Similar ways when heated or when reacted with acids
- Metal carbonates decompose to the Metal oxide and carbon dioxide when they are heated strongly enough
- A bunsen burner flame cannot get hot enough to decompose sodium carbonate or potassium carbonate
- all carbonates react with acids to produce a salt, water and carbon dioxide gas. limestone is damaged by acid rain because the calcium carbonate in the limestone reacts with acids in the rain
- calcium hydroxide solution is called limewater. limewater is used to test for carbon dioxide. the limewater turns cloudy because it reacts with carbon dioxide to produce insoluble calcium carbonate
3 of 23
C1.2.3 The "Limestone Reaction Cycle"
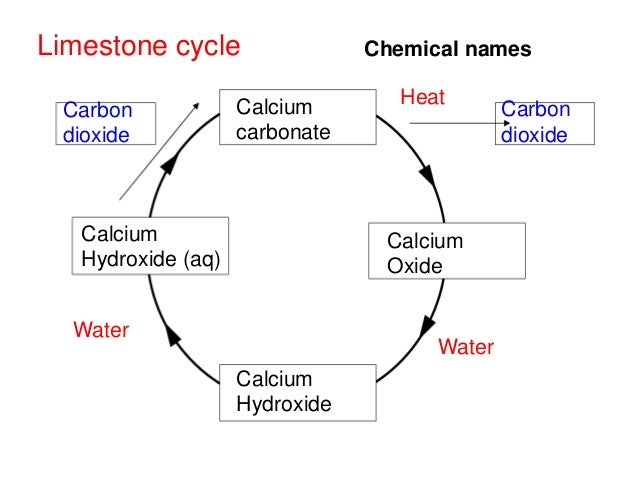
- Calcium hydroxide is an alkali and so it can be used to neutralise acids. For example, it is used by farmers to neutralise acidic soils and in industry to neutralise acidic gases.
- Calcium hydroxide is not very soluble in water but dissolves slightly to make limewater
4 of 23
C1.2.4 Cement and Concrete
- To make cement, limestone is mixed with clay and heated strongly in a kiln. It is then ground up to make a fine powder
- cement is mixed with sand and water to make mortar. It is used to hold bricks together (like glue)
- We make concrete by mixing cement with sand, aggregate, and water. The mixture can be poured into moulds before it sets to form a hard solid.
Pros of limestone quarrying: Jobs, More business for locals, Roads improved
Cons of limestone quarrying: Destroys habitats, dust, sound pollution, visual pollution
5 of 23
C1.3.1 Extracting metals
- Rocks that contain enough of a metal are known as ores
- Gold can be separated from rocks by physical methods but most have to be extracted by chemical reactions
- metals can be extracted from compounds by displacement using a more reactive element. metals which are less reactive than carbon can be extracted from the oxides by heating with carbon. a reduction reaction takes place as carbon removes the oxygen from the oxide to produce the metal. this method is used commercially
- many ores contain iron oxide which can only be reduced at high temperatures in a blast furnace using carbon. the impurities make it hard and brittle and so it has only a few uses as cast iron. removing all the carbon and impurities makes pure iron but this is too soft
- steel is an alloy of iron and carbon. it is strong and has properties for specific uses.
6 of 23
C1.3.2 Iron and Steels
- Low carbon steels are easily shaped. high carbon steels are hard
- stainless steels contain lots of other metals. they resist corrosion
- aluminium has low density although it's high in the reactivity series, it is resistant to corrosion
- aluminium is more reactive than carbon and so its oxide cannot be reduced using carbon
- extracted by electrolysis of molten aluminium oxide. requires high temp, a lot of electricity. it is expensive to extract
- pure aluminium is not strong, but aluminium alloys are stronger and harder
- titanium is resistant to corrosion and very strong. low density compared to other metals
- titanium oxide can be reduced be carbon, but the metal reacts with carbon making it brittle
- titanium is extracted from its ore involving several stages and lots of energy. it is expensive
- titanium used for hip joint, aluminium used for aircraft
7 of 23
C1.3.4 Extracting Copper
- copper is extracted from copper-rich ores by smelting- heating ore strongly in a furnace
- smelting produces impure copper which is purified by electrolysis. lots of heat and energy is required
- copper-rich ores are a limited resource. scientists are testing low-grade ours which have less environmental impact than smelting
- phytomining uses plants to absorb copper compounds from the ground. plants burn producing ash which copper can be extracted from
- bioleaching uses bacteria to produce solutions containing copper compounds
- solutions of copper compounds can be reacted with a metal that is more reactive than copper, such as scrap iron, to displace the copper
- copper can be extracted from solutions of copper compounds by electrolysis with carbon electrodes
8 of 23
C1.3.6 Metallic Issues
- mining for metal ores involves digging up and processing large amounts of rocks. this produces large amounts of waste material and affects large areas of the environment
- recycling metals: saves energy needed to extract the metal, resources because less ore needs to be mined, less fossil fuels is needed to provide the energy to extract the metal from its ore
- benefits of metals in construction: strong, bend into shape, flexible wires, good electrical conductors
- Cons of metals in construction: obtaining metals uses limited resources and causes pollution, more expensive than the likes of concrete, iron and steel can rust
9 of 23
C1.4.1 Fuels from crude oil
- distillation can be used to separate mixtures of liquids.
- fractions- hydrocarbons with similar boiling points separated from crude oil.
- the larger the molecule, the higher the boiling point of the hydrocarbon.
- the crude oil is vaporised and fed into a fractioning column. this is a tall tower that is hot (350') at the bottom and cool (50') at the top.
- the top is short-chain hydrocarbons and the bottom is residue filled with long-chain hydrocarbons.
- the hydrocarbons condense to liquids when they reach the level that is at their boiling point. different liquids collect on the trays at different levels and there are outlets to collect the fractions.
- with low boiling ranges have low viscosity so they are runny liquids. they are very flammable so they ignite easily. they also burn with clean flames, producing little smoke. this makes them very useful as fuels.
10 of 23
C1.4.3 Burning Fuels
- when pure hydrocarbons burn completely they are oxidised to carbon dioxide and water. however, the fuels we use are not always burned completely. they may also contain other substances.
- in a limited supply of air, incomplete combustion may produce carbon monoxide. carbon may also be produced and some of the hydrocarbons may not burn. this produces solid particles that contain soot (carbon) and unburnt hydrocarbons called particulates.
- these particulates cause global dimming
- most fossil fuels contain sulfur which when burned produces sulfur dioxide causing acid rain as nitrogen and sulfur dissolve in water droplets and react with oxygen in the air to produce the acid rain
- at high temperatures produced when fuels burn, oxygen and nitrogen in the air may combine to form nitrogen oxides which cause acid rain
- exhaust systems of cars are fitted with catalytic converters to remove carbon monoxide and nitrogen oxides. sulfur can be removed from fuels before they are supplied to users
11 of 23
C1.4.5 Alternative Fuels
- Biodiesel can be made from veg oils
- pros of biodiesel: little contribution to carbon dioxide levels because it was carbon neutral taking in CO2 for photosynthesis
- Cons of biodiesel: the land could have been used as farmland
- ethanol made from sugar is a biofuel
- pros of hydrogen fuel: only produces water
- Cons of hydrogen fuel: it's gas so it takes up a large volume to be stored
- it can be produced from water by electrolysis but this requires large amounts of energy
12 of 23
C1.5.1 Cracking hydrocarbons
- large hydrocarbon molecules can be broken down into smaller molecules by a process called cracking
- 2 types of cracking: heating mixture of hydrocarbon vapours and steam in high temp, passing hydrocarbon vapours over a hot catalyst
- alkanes are saturated hydrocarbons with smaller molecules and are more useful as fuels CnH2n+2
- alkenes are unsaturated hydrocarbons wth smaller molecules too and have a double bond between two carbon atoms and this makes them more reactive than alkanes. alkenes react with bromine water turning it from orange to colourless CnH2n
- polymers are made from many small molecules (monomers) joined together(polymerisation).

13 of 23
C1.5.3 New And Useful Polymers
- new polymer materials for dental fillings have been developed to replace fillings that contain mercury.
- light sensitive plasters to cover up wounds so it can be removed easily
- shape memory polymers change back to their original shape when the temperature or other conditions are changed. for example, a smart polymer is a material used for stitching wounds that change shape when heated to body temperature
- fibres used to make fabrics can be coated with polymers to make them waterproof and breathable
- plastic used to make drink bottles can be recycled to make polyester clothing.
- many polymers are not biodegradable
- some plastics contain cornstarch, microorganisms break down the cornstarch and leaves the plastic in small pieces that can be mixed with soil
- recycling plastics is hard to sort
14 of 23
C1.5.5 Ethanol
- ethanol made by fermenting sugar using enzymes in yeast.
- enzymes in the yeast cause the sugar to convert to ethanol and carbon dioxide which is used to make alcoholic drink.
- ethanol can be made by the hydration of ethene.
- ethene is reacted with steam at a high tempreture in the presence of a catalyst. ethene is obtained from crude oil by cracking.
- ethanol produced by fermentation uses a renewable resource, sugar from plants
- fermentation is done at room temp. but, it can only produce a dilute aqueous solution of ethanol. the ethanol must be separated from the solution by fractional distillation to give pure ethanol
- ethanol produced from ethene uses a non-renewable resource, crude oil
- the reaction can be run continuously and produces pure ethanol, but requires a high temp
15 of 23
C1.6.1 Extracting Veg Oil
- some seeds, nuts and fruits are rich in veg oils which can be extracted by crushing and pressing the plant material followed by removing water and other impurities. some oils are extracted by distilling plant material mixed with water. the oil can now be separated from the mixture of water and oil
- veg oils provide a lot of energy and important nutrients. veg oils produce lots of energy when they burn in the air and so are used in biofuels and biodiesel
- molecules of veg oils are hydrocarbon chains. double bonds are unsaturated C=C. several bonds are called polyunsaturated. unsaturated oils turn orange bromine water colourless
16 of 23
C1.6.2 Cooking with veg oils
- boiling point of veg oils higher than water so food is cooked at higher temp resulting in cooking faster. it also changes the flavour, colour and texture of the food. some of the oils are absorbed therefore energy content of food increases.
- unsaturated oils can react with hydrogen to make C=C bond single C-C, the reaction is hydrogenation. the reaction is 60', nickel catalyst
- hydrogenated oils have higher melting points because they are saturated. the reaction is also called hardening because the hydrogenated oils are solids at room temperature. they can be used in spread, pastries and cakes that require solid fats
- emulsions are opaque and thicker than the oil and water they are made from. this improves texture, appearance and ability to coat and stick to solids. milk, cream and salad dressings are emulsions.
- emulsifiers help stop the oil and water from separating into layers.
- hydrophillic- "head" attracted to water
- hydrophobic- "tail" attracted to oil
17 of 23
C1.6.4 Food Issues
- veg oils are high in energy and contain important nutrients. they contain unsaturated fats which are better for your health than saturated fats.
- animal fats and hydrogenated veg oils contain saturated fats and are used mainly in foods. saturated fats linked to heart disease.
- emulsifiers make food smoother and tastier, therefore, you are more likely to eat more which is not good for your health
18 of 23
C1.7.1 Structure of the Earth
- the surface of the earth is a thin, solid crust.
- mantle is under the crust and is almost entirely solid but parts of it can flow very slowly. it goes halfway to the centre of the Earth.
- the core is half the diameter of the Earth. it has the majority of magnetic metals iron and nickel. it has a liquid outer part and solid inner part.
- atmosphere surrounds Earth.
- the crust, ocean and atmosphere provide us with raw materials.
- tectonic plates move a few cm's a year because of convection currents in the mantle. this is from the decay of radioactive elements heating up the mantle.
- where the plates meet, huge forces build up and earthquakes, volcanoes or mountains form.
- Wegener had the idea of continental drift in 1915. other scientists disagreed as he couldn't explain why the continents moved. 1960's scientists found new evidence and the theory was developed
19 of 23
C1.7.3 The Earth's Atmosphere In The Past
- Earth formed about 4.5 billion years ago. in the first billion years, the surface was covered with volcanoes which released carbon dioxide, water vapour and nitrogen.
- as the earth cooled, water vapour condensed to form oceans. the early atmosphere was mainly carbon dioxide with some water vapour. some believe there was nitrogen and possibly some methane and ammonia
- in the next 2 billion years bacteria, algae and plants evolved. algae and plants used carbon dioxide for photosynthesis which released oxygen. as the number of plants increased the amount of carbon dioxide in the atmosphere decreased and oxygen increased.
20 of 23
C1.7.4 Life On Earth
- Plants that produced oxygen most likely came from simple organisms like plankton and algae in the ancient oceans. there's insufficient evidence to prove how life began.
- Miller-Urey experiment was what they thought was in the early atmosphere. they used a mixture of water, ammonia, methane and hydrogen and a high voltage spark to stimulate lightning. after a week they found amino acids which are building blocks for proteins.
- another theory suggests that these organic materials formed a "primordial soup" and that the amino acids in this mixture combined to make proteins from which life began. No evidence to prove theory.
21 of 23
C1.7.5 Gases In The Atmosphere
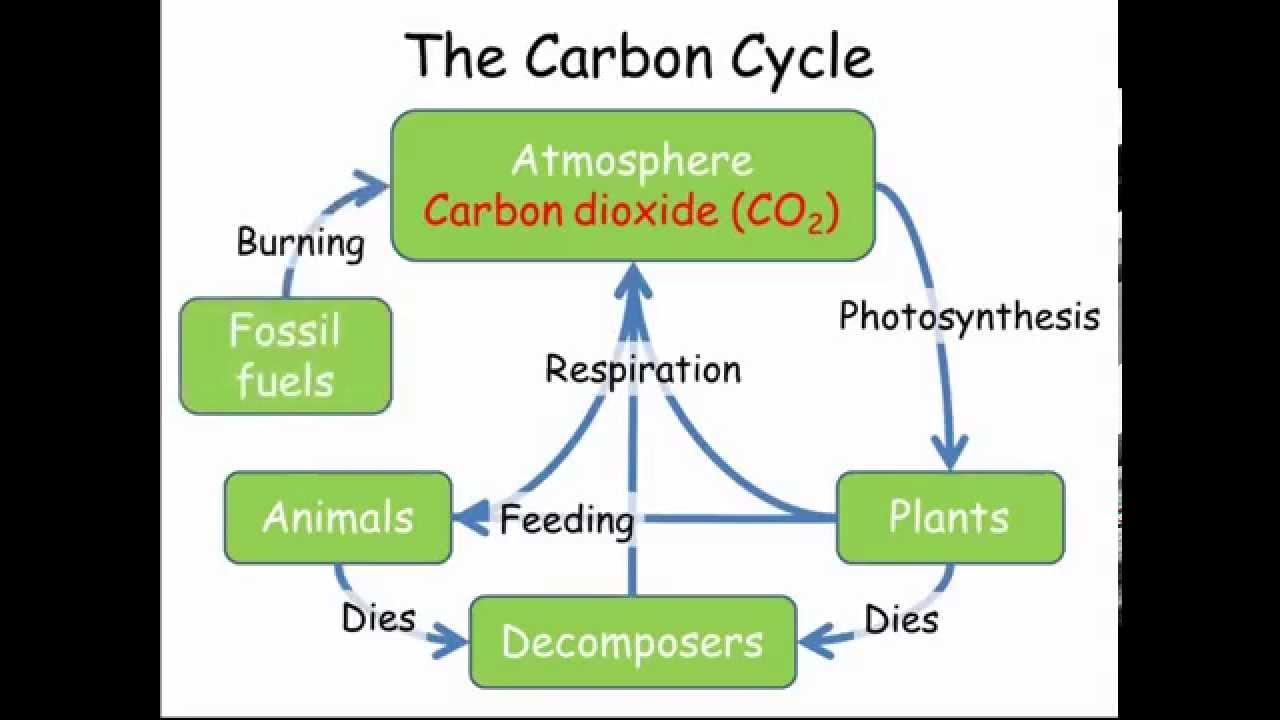
- Plants take in CO2 from atmosphere, animals eat plant, there remains contain carbon found in the sedimentary rocks and fossil fuels. limestone formed from the shells and skeletons of marine organisms. fossil fuels contain carbon and hydrogen from plants and animals.
- carbon dioxide dissolves in oceans and some formed insoluble carbonate compounds that were deposited on the seabed and became sedimentary rocks.
- nitrogen 78% Oxygen 21% argon 0.9% carbon dioxide 0.04%
- gases can be separated from liquid air by fractional distillation. this is done in industry to get pure oxygen and liquid nitrogen. air is cooled to -200' and fed into the fractional distillation column. nitrogen gas out the top, liquid oxygen out the bottom.
22 of 23
C1.7.6 Carbon dioxide in the atmosphere
- carbon dioxide dissolves in water, particularly the oceans, and reactions of inorganic carbonate compounds are also important in maintaining a balance.
23 of 23
Related discussions on The Student Room
- Edexcel Past Papers »
- Entry requirements university of Hertfordshire »
- Applied Science Unit 3 investigation skills »
- Maths for economists - constrained maximisation questoin »
- Should I repeat Chemistry exams? »
- BTEC Level 3 Applied Science Unit 1 May 2022 Exams »
- Applied Science Unit 1 Exam 2023 biology »
- Edexcel chemistry unit 2 mixed questions »
- Bangor University GCSE Revision guides? »
- How hard is the Btec level 3 extended certificate in applied science? »
Similar Chemistry resources:
1.0 / 5 based on 1 rating
0.0 / 5
3.0 / 5 based on 1 rating
0.0 / 5
0.0 / 5
0.0 / 5
0.0 / 5
0.0 / 5
0.0 / 5
3.0 / 5 based on 1 rating
Comments
No comments have yet been made