BIOLOGICAL MOLECULES
- Created by: charlotte.jakes7
- Created on: 21-05-18 13:39
CARBOHYDRATES - MONOSACCHARIDES
MONOSACCHARIDES.
- sweet tasting, soluble substances with general formula (CH2O)n where n is any number from one to 7
- monomer units of polysaccharides
TEST FOR REDUCING SUGARS.
1. 2cm^3 of food sample + equal volume Benedict's reagent
3. heat gently in water bath for five minutes
POSITIVE RESULT: orange-brown colour change
DISACCHARIDES - two monosaccharides joined by a glycosidic bond from condensation reaction.
- glucose + glucose = maltose
- glucose + fructose = sucrose
- glucose + galactose = lactose
CARBOHYDRATES - POLYSACCHARIDES
TEST FOR NON-REDUCING SUGARS.
1. test for reducing first
2. add 2cm^3 sample to equal volume dilute hydrochloric acid, heat in gently boiling water bath for five mins
3. add sodium hydrogencarbonate slowly in order to give alkaline pH
4. add 2cm^3 Benedict's solution and heat in gently boiling water bath for five mins
RESULT: orange-brown colour change
POLYSACCHARIDES.
- polymers of many monosaccharide monomer joined by glycosidic bonds from condensation
- large + insoluble - suitable for storage
TEST FOR STARCH.
1. 2cm^3 food sample + two drops of iodine solution + shake
RESULT: blue-black colour change
STARCH
STRUCTURE.
- polysaccharide found in plants, especially seeds and storage organs (e.g. potato tubers)
- made up of chains of a-glucose linked by glycosidic bonds from condensation reactions
- may be branches or unbranched - unbrached is wound into tight coil, making molecule compact
WHY IS IT A GOOD ENERGY STORE?
- insoluble - doesn't affect water potnetial
- large + insoluble - doesn't diffuse out of cells
- compact - lots stored in small space
- forms a-glucose when hydrolysed - easily transported + used in respiration
- branched form has many ends - enzymes can act simultaneously for rapid release of glucose
GLYCOGEN
STRUCTURE.
- found in granules of the muscles and liver of animals
- similar to starch but has shorter, more highly branched chain
WHY IS IT A GOOD ENERGY STORE?
- insoluble - doesn't affect water potential
- insoluble - doesn't diffuse out of cells
- compact - lots stored in small space
- more highly branched than starch - enzyme action occurs simultaneously, rapidly releasing glucose for respiration (animals have high metabolic rate and thus higher respiratory rate due to activity)
CELLULOSE
STRUCTURE.
- long straight chains of B-glucose
- parallel chains joined by hydrogen bond cross-linkages - gives overall strength
- grouped into microfibrils and fibres
FUNCTION.
- major component in cell walls
- provides rigidity to plant cell
- prevents cell from bursting due to osmotic pressure - exerts inward pressure that prevents further influx of water
- makes cells turgid - push against one another to make plant semi rigid (e.g. leaves + stems)
LIPIDS
- contain carbon, hydrogen and oxygen
- proportion of oxygen to carbon and hydrogen is smaller than in carbohydrates
- insoluble in water
- soluble in organic solvents
ROLES.
- as phospholipids in cell-surface membranes to allow flexibility + transfer of lipid-soluble substances
- source of energy - release twice as much energy as carbohydrates when oxidised
- waterproofing - insoluble in water so allow waxy cuticles + oily secretion on mammals
- insulation - slow conductors of heat, retain body heat and act as electrical insulators
- protection - stored around delicate organs (e.g. kidneys)
TRIGLYCERIDES
STRUCTURE.
- three faty acids combined with glycerol through ester bonds from condensation reactions
- SATURATED: no double bonds
- MONO-UNSATURATED: one double bond
- POLYUNSATURATED: more than one double bonds
PROPERTIES.
- high ratio of energy storing C-H bonds to carbon atoms
- low mass to energy ratio - reduces mass for animals to carry aroun
- large + non polar - insoluble, don't affect water potential
- high ratio of hydrogen to oxygen atoms - release water when oxidised so provide source of water in dry conditions
PHOSPHOLIPIDS
STRUCTURE.
- one of fatty acid molecules replaced by phosphate
- hydrophilic head - attracted to water but not fat
- hydrophobic tail - repelled by water but not fat
- therefore polar
PROPERTIES.
- polar - form bilayers within aqueous environments to form cell-surface membranes of cells
- hydrophilic heads hold at surface of cell-surface membrane
- allows them to combine with carbohydrates to form glyolipids - important in cell-cell recognition

PROTEINS
AMINO ACIDS.
- monomer units of polypeptides
- amino -NH2 group
- carboxyl group
- hydrogen atom
- R group - variety of chemical groups
FORMING POLYPEPTIDES.
- amino acids joined by peptide bonds in condensation reactions - -OH from carboxyl group and -H from amino group
PROTEIN STRUCTURE
PRIMARY.
- sequence of amino acids in polypeptide chain determined by DNA
- determines where bonds form - determines ultimate shape and function of protein
SECONDARY.
- hydrogen bonds form between positive H of -NH group and negative O of -OH group
- twist primary structure into 3D a-helix
TERTIARY.
- formation of ionic bonds, hydrogen bonds + disulfide bridges
- gives complex 3D sturcture
QUATERNARY.
- combination of different protein and non-protein groups
ENZYME ACTION
- lower the activation energy of reactions by providing alternate pathway to reactions
- allows human body temperature to be maintained at 37 degrees
STRUCTURE.
- globular proteins with 3D specific shapes
- active site - specific functional region made up of small number of amino acids
- substrace fits into depression formed by active site - enzyme-substrate complex
INDUCED FIT MODEL.
- enzyme is not initially truly complementary to substrate
- as substrate binds, enzyme changes shape to mould around
- enzyme puts strain on substrate, weakening the bonds within and making them easier to break
ENZYMES AND REACTIONS
MEASURING ENZYME-CATALYSED REACTIONS.
- rate of formation of products
- rate of disappearance of substrate
ENZYMES + RATE.
- lots of substrate = lots of contact = lots of complexes = lots of hydrolysis
- substrate decreases as it is broken down, product increases
- less substrate and more product makes it difficult for complexes to form
- less hydrolysis per unit time
- eventually all substrate used up
ENZYMES + CONDITIONS
TEMPERATURE.
- as temp increases, energy increases, more complexes + hydrolysis per unit time
- temp too high = bonds within begin to break, changing active site's shape until it is no longer complementary + becomes denatured
BODY TEMPERATURE.
- higher than 37 degrees would increase metabolic rate but additional food would be needed to maintain temp
- other proteins would be denatured at higher temperetures
- any further rise in temperature could denature the enzymes
pH.
- pH other than optimum alters charges on amino acids of binding site so alters substrate binding, can cause bonds to break unravelling tertiary structure
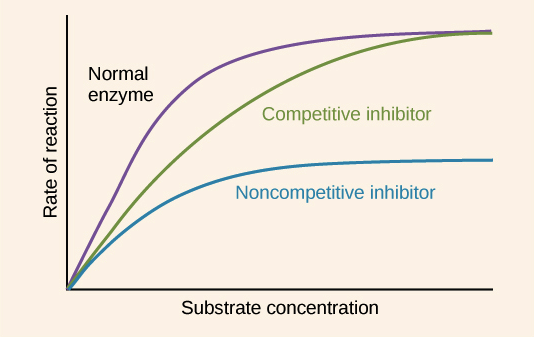
ENZYMES + CONCENTRATION
ENZYME CONCENTRATION.
- low - too few enzyme molecules for all substrate molecules to fill
- intermediate - all active sites filled at any given time
- high - no more substrate added so too many active sites
SUBSTRATE CONCENTRATION.
- low - too few substrate molecules to occupy active sites
- intermediate - all active sites filled at any given time
- high - not enough active sites for substrate fo fill
NUCLEIC ACIDS
NUCLEOTIDE STRUCTURE.
- pentose sugar
- phosphate group
- nitrogen containing organic base
- joined by condensation reactions forming phosphodiester bonds between sugars and phosphates
- forms dinucleotides and polynucleotides
RNA STRUCTURE.
- single, short polynucleotide chain
- pentose sugar is ribose
- organic bases - adenine, uracil, cytosine, guanine
- can transfer genetic information from DNA to ribosomes, make up ribosomes and bring amino acids to mRNA for protein synthesis
DNA REPLICATION
SEMI-CONSERVATIVE.
REQUIREMENTS...
- four types of nucleotide each with the four bases
- both strands of DNA to act as a template
- enzyme DNA polymerase
- source of chemical energy
PROCESS.
1. DNA helicase breaks hydrogen bonds linking base pairs
2. double helix separates into two strands and unwinds
3. exoposed polynucleotides act as templates upon which complementary free nucleotides bind by base pairing
4. adjacent nucleotides joined in condensation reactions by DNA polymerase
5. two new DNA molecules contain one 'old' and one 'new' strand
MESELSOHN + STAHL
WORK BASED ON...
- all bases in DNA contain nitrogen
- nitrogen has lighter 14N and heavier 15N isotopes
- bacteria incorporate nitrogen from their growing medium into their DNA
PROCEDURE.
1. grew original bacteria in 15N medium
2. transferred bacteria to 14N for single replication
3. centrifuged DNA molecule - nearer the top, lighter the stand
FINDINGS.
first generation: one heavy, one light strand = middle of tube
second generation: one light molecule at top, one mixed at middle
ENERGY + ATP
STRUCTURE.
- adenine - nitrogen containing organic base
- ribose - pentose sugar
- three phosphates
STORAGE OF ENERGY.
- bonds between phosphate groups are unstable - low activation energy
- release considerable amount of energy when broken
- ATP + H2O = ADP + Pi + Energy
- hydrolysis reaction catalysed by ATP hydrolase
SYNTHESIS OF ATP
- hydrolysis is a reverisble reaction
- ADP + Pi = ATP + H2O
- catalysed by ATP synthase in a condensation reaction
- occurs in chlorophyll-containing plant cells during photosynthesis, during respiration, and during phosphorylation where phosphate groups are transferred from donor molecules
ROLES OF ATP
WHY IS IT A GOOD ENERGY DONOR?
- each ATP molecule releases less energy than glucose - released in smaller, more manageable quantities
- hydrolysis of ATP to ADP is single step reaction - shorter proces than glucose breakdown
WHERE IS IT USED?
- metabolic processes - energy needed to build up macromolecules
- movement - energy for muscle contraction
- active transport - energy to change shape of carrier proteins
- secretion - energy to form lysosomes
- activation of molecules - inorganic phosphate can phosphorylate other compounds to lower their activation energy and make them more reactive
WATER
PROPERTIES.
- dipolar - oxygen has partial negative, hydrogen has partial positive
- hydrogen bond - opposite poles attract causing molecules to stick together
- specific heat capacity - hydrogen bonding means more energy required to heat, gives high specific heat capacity that acts as a buffer against sudden temperature changes in aquatic and terrestrial environments
- latent heat of vaporisation - lots of energy required to evaporate 1g of water so water is an effective means of cooling
- cohesion - hydrogen bonding allows water to be pulled through a tube e.g in xylem
- surface tension - when molecules meet air they are pulled back into the body of water
IMPORTANCE.
- metabolism - hydrolysis, aqueous medium for reactions, raw material in photosynthesis
- solvent - gases (O2, CO2), wastes, inorganic ions + small hydrophilic molecules, enzymes
- cooling mediu, not easily compressed to provides support in skeletons of insects and plants, transparent to allow aquatic plants to photosynthesise + light to reach the retina
Related discussions on The Student Room
- Any good youtube channels for Bio + Chem a levels? »
- Do I need to know how to draw structures for carbohydrates? (AQA A Level Bio) »
- Paper 3 AQA a Level biology »
- 25 mark essay question »
- BTEC applied science Unit 10 »
- Access to Science course »
- AQA A Level Biology »
- exams 2022 »
- Biology help please »
- Biology AS Question Help »
Comments
No comments have yet been made