B1 - Cell Level Systems
- Created by: munchlee142002
- Created on: 20-10-17 17:42
Organisms can be Eukaryotes or Prokaryotes
- Eukaryotes (e.g. all animals and plants) are made from complex cells called eukaryotic cells.
- Prokaryotes (e.g. bacteria) are smaller and simpler cells called prokaryotic cells.
- Both types of cells contain sub-cellular structures - parts of cells that each have a specific function.
The structures within an animal cell
- Nucleus - contains DNA (genetic material) in the form of chromosomes that controls the cell's activities.
- Cytoplasm - gel-like substance where most of the chemical reactions happen.
- Mitochondria - these are the site of cellular respiration and contain the enzymes needed for the reactions involved.
- Cell membrane - holds the cell together and controls what goes in and out by providing a selective barrier. They also contain receptor molecules that are used for cell communication e.g. by hormones
- Ribosomes - Where proteinsynthesis occurs
The structures within a plant cell
Plant cells include everything animals cells have (nucleus, cytoplasm, mitochondria, cytoplasm and ribosomes) as well as extras:
- Rigid cell wall - made of cellulose, gives support for the cell.
- Chloroplasts - where photosynthesis occurs. They contain a green substance called chlorophyll.
Structures within prokrayotic cells
- Chromosomal DNA - (one long circular chromosome) controls the cell's activities and replication. It floats free in the cytoplasm (not in a nucleus).
- Plasmids - small loops of extra DNA that aren't part of the chromosom. Plamids contain genes for things like drug resistance, and can be passed between bacteria.
- Cell membrane - controls what goes in and out. The cell is also supported by a cell wall.
Cells are studied using microscopes
- Microscopes use lenses to maginfy images (make them look bigger).
- They also increase the resolution of an image. This means they increase the detail you can see. Resolution is how well a microscope distinguishes between two points that are close together.
- Light microscopes were invented in the 1590s. They let us see things like nuclei and chloroplasts.
- Electron microscopes were invented in the 1930s. They let us see smaller things in more detail like the internal structure of mitochondria. This allows us to have a much greater understanding of sub-cellular structures. Only electron microscopes will let us see things as tiny as plasmids or viruses.
- Transmission electron microscopes (TEMs) have a higher magnification and resolution than light microscopes but they're not portable, they're expensive and it's a complicated process to prepare specimens for use (they can't be used to look at living tissue, unlike light microscopes).
Parts of a light microscope
The main parts of a light microscope and what they do:
- Eyepiece lens - looked through to see the image and also magnifies the image
- Objective lens - magnifies the image. Usually there are three different objective lenses (e.g. x4, x10 and x40).
- Stage - supports the slide
- Clip - holds the slide in pace
- Handle - to carry the microscope with
- Lamp - shines light through the slide so the image can be seen more easily
- Focusing knobs - move the stage up and down to bring the image into focus.
Specimens need to be prepared before investgation
- Your specimen (the sample you're looking at) needs to let light through it for you to be able to see it clearly - if you've got a thick specimen, you'll need to take a thin slice of it to start with.
- Next, take a clean slide (a ***** of clear glass or plastic) and use a pipette to put one drop of water or mountant (a clear, gloopy liquid) in the middle of it - this will secure the specimen in place.
- Use tweezers to place your specimen on the slide.
- Add a drop of stain if needed - if your specimen is completely transparent or colourless, a drop of stain is added to make the specimen easier to see. Different stains are used to highlight different structures or tissues. For example, eosin is used to stain cytoplasm and methylene blue stains DNA.
- Place a cover slip (a square of thin, transparent plastic or glass) at one end of the specimen, holding it at an angle with a mounted needle.
- Carefully lower the cover slip onto the slide. Press it down gently with the needle so that no air bubbles are trapped under it.
Specimens ready for viewing
- Start by clipping the slide containing your specimen onto the stage.
- Select the lowest-powered objective lens (e.g. the one that produces the lowest magnification).
- Use the coarse adjustment knob to move the stage up to just below the objective lens. Then, looking down the eyepiece, move the stage downwards (so you don't accidentally crash the slide into the lens) until the specimen is just about in focus.
- Then, still looking down the eyepiece, adjust the focus with the fine adjustment knob, until you get a clear image of your specimen.
- If you need to see your specimen with greater magnification, swap to a higher-powered objective lens and refocus.Once you're happy with that you can see, you can make a scientific drawing of the specimen.
DNA - a double helix of paired bases
DNAcontains all of an organism's genetic material - the chemical instructions it needs to grow and develop. DNA is arranged into chromosomes.
- Chromosomes are long molecules of coiled up DNA. The DNA is divided up into short sections called genes.
- DNA is a double helix (a double-stranded spiral). Each of the two DNA strands is made up of lots of nucleotides joined together in a long chain - this makes DNA a polymer.
- Each nucleotide contains a small molecule called a "base".DNA has just four different bases.
- The bases are A (adenine), C (cytosine), G (guanine) and T (thymine).
- Each base forms cross links to a base on the other strand. This keeps the two DNA strands tightly wound together.
- A always pairs up with T, and C always pairs up with G. This is called complementary base-pairing.
Nucleotide - sugar, phosphate group and a base
- Each DNA nucleotide has the same sugar and a phosphate group. The base on each nucleotide is the only part of the molecule that varies (i.e it's either A, C, G or T).
- The base is attached to the sugar.
DNA is a polymer
- Polymers are large, complex molecules composed of long chains of monomers joined together.
- Monomers are small, basic molecular units.
- DNA is a polymer made up of nucleotide monomers.
Enzymes control cell reactions
- Cells have thousands of different chemical reactions going on inside them all the time - like respiration, photosynthesis and protein synthesis. Together these make up the cell's metabolism.
- These reactions need to be carefully controlled - to get the right amounts of substances and keep the organism working properly.
- You can usually make a reaction happen more quickly by raising the temperature. This would speep up the useful reactions but also the unwantedbones too. There's also a limit to how far you can raise the temperature inside a living creature before its cells start getting damaged.
- So living things produce enzymes, which act as biological catalysts. A catalyst is a substance that speeds up a reaction, without being changed or used up in the reaction itself.
- Enzymes reduce the need for high temperatures and we only have enzymes to speed up the useful chemical reactions in the body.
- Every different biological reaction has its own enzyme especially for it.
- Each enzyme is a protein coded for by a different gene, and has a unique shape which it needs to do its job.
Enzymes are very specific
- Chemical reactions usually involve things either being split apart or joined together
- The substrate is the molecule changed in the reaction.
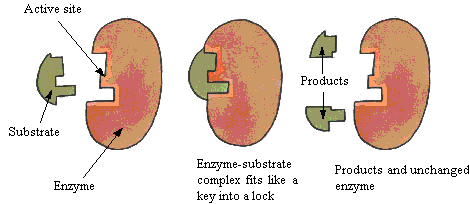
- Every enzyme has an active site - the part where it joins on to its substrate to catalyse the reaction.
- Enzymes are really picky - they usually only work with one substrate. Enzymes have a very high specificy for their substrate.
- This is because, for the enzyme to work, the substrate has to fit into the active site. If the substrate's shape doesn't match the active site's shape, then the reaction won't be catalysed. This is called the 'lock and key' hypothesis, because the substrate fits into the enzyme just like a key fits into a lock.
Lock and Key hypothesis
Enzymes like it warm but not hot
- Changing the temperature changes the rate of an enzyme-controlled reaction.
- A higher temperature increases the rate at first. The enzymes and the substrate move about more, so they're more likely to meet up and react. But if it gets too hot, some of the bonds holding the enzyme together break. This make the enzyme become denatured - it loses its shape and the substrate doesn't fit the active site any more. This means the enzyme can't catalyse the reaction and the reaction stops. The enzyme is denatured irreversibly - it won't go back to its normal shape if things cool down again.
- Each enzyme has its own optimum temperature when the reaction goes its fastest. This is the temperature just before it gets too hot and starts to denature. The optimum temperature for the most important human enzymes is 37°C - the same temperature as our bodies.
Enzymes like the right pH
- The pH has an effect on enzymes. If it's too high or too low, it interferes with the bonds holidng the enzyme together. This changes the shape of the active site and can irreversibly denature the enzyme.
- All enzymes have an optimum pH that they work best at. It's often neutral pH 7, but not always. For example, pepsin is an enzyme used to break down proteins in the stomach. It works best at pH 2, which means it's well-suited to the acidic conditions in the stomach.
Enzyme concentration affects the rate of reaction
- The more enzyme molecules there are in a solution, the more likely a substrate molecule will meet up with one and join with it. So increasing the concentration of the enzyme increases the rate of reaction.
- But if the amount of substrate is limited, there comes a point when there are more than enough enzyme molecules to deal with all the available substrate, so adding more enzyme has no further effect.
Substrate concentration affects the rate
- The higher the substrate concentration, the faster the reaction - it's more likely the enzyme will meet up and react with a substrate molecule.
- This is only true up to a point. After that, there are so many substrate molecules that the enzymes have about as much as they can cope with (all the active sites are full), and adding more makes no difference.
Enzymes optimum temperature
The graph for optimum temperature - where the enzyme is most active:
Enzymes optimum pH
A graph to show an enzymes optimum pH:
Enzyme concentration graph
A graph that shows how enzyme concentration affects the rate of reaction:
Substrate concentration graph
A graph showing how the substrate concentration affects the rate up to a point:
How temperature affects enzyme activity
There are a couple of different ways to investigate how temperature affects enzyme activity. You can also adapt these experiments to measure variables other than temperature. For example:
- To investigate the effect of pH, add a buffer solution with a different pH level to a series of different tubes containing the enzyme-substrate mixture.
- Vary the initial concentrations of the substrate to investigate the effect of substrate concentration.
- Vary the initial concentrations of the enzyme to investigate the effect of enzyme concentration.
Measure how fast a product appears
- The enzyme catalase catalyses the breakdown of hydrogen peroxide into water and oxygen.
- You can collect the oxygen and measure how much is produced in a set time.
- Use a pipette to add a set amount of hydrogen peroxide to a boiling tube. Put the tube in a water bath at 10°C.
- Set up the rest of the appearance as shown. Add a source of catalase (e.g 1cm cubed of a potato) to the hydrogen peroxide and quickly attach the bung.
- Record how much oxygen is produced in the first minute. Repeat three times and calculate the mean.
- Repeat at 20°C, 30°C and 40°C.
- Control any variables (e.g. the pH, the potato used, the size of potato pieces, etc.) to make it a fair test.
- Calculate the mean rate of reaction at each temperature by dividing the mean volume of oxygen produced (in cm cubed) by the time taken (e.g. 60s).
Measure how fast a substrate disappears
- The enzyme amylase catalyses the breakdown of starch and maltose.
- It's easy to detect starch using iodine solution - if starch is present, the idoine solution will change from browny-orange to blue-black.
- Set up the apparatus. Put a drop of iodine solution into each well on the spotting tile. Every ten seconds, drop a sample of the mixture into a well using a pipette. When the iodine solution remains browny-orange (i.e starch is no longer present) record the total time taken.
- Repeat with the water bath at different temperatures to see how it affects the time taken for the starch to be broken down. Remember to control all the variables each time.
You could improve the accuracy of this experiment by using a colorimeter - a piece of elctronice equipment that measures the strength of a coloured solution so measurements aren't just based on somebody's judgement of when the colour has changed.
Respiration is not "breathing in and out"
- Respiration is the process of transferring energy from the breakdown of glucose (a sugar). It goes on in every cell in all living organsims, all the time - it's a universal chemical process.
- The energy transferred by respiration can't be used directly by cells - so it's used to make a substance called ATP. ATP stores the energy needed for many cell processes.
- Respiration is controlled by enzymes, so the rate of respiration is affected by both temperature and pH. It's an exothermic reaction - it transfers energy to the environment (by heat).
- Cells can respire using glucose as a substrate, but organisms can also break down other organic molecules (such as other carbohydrates, proteins and lipids) to use as substrates for respiration.
Aerobic respiration needs oxygen
- Aerobic respiration is what happens when there's plenty of oxygen available.
- "Aerobic" just means "with oxygen" and it's most efficient way to transfer energy from glucose. Aerobic respiration produces lots of ATP - 32 molecules per molecule of glucose.
- This is the type of respiration that you're using most of the time.
- Here is the equation for aerobic respiration:
glucose + oxygen → carbon dioxide + water (+ energy)
C6H12O6 + 6O2 → 6CO2 + 6H2O
Anaerobic respiration doesn't use oxygen
- "Anaerobic" just means "without" oxygen.
- It's not the best way to transfer energy from glucose because it tranfers much less energy per glucose molecule than aerobic respiration - just 2 molecules of ATP are produced.
- The process of anaerobic respiration is slightly different in different organisms.
Animals produce lactic acid during anerobic
- When you do really vigorous exercise your body can't supply enough oxygen to your muscles for aerobic respiration - even though your heart rate and breathing rate increase as much as they can. Your muscles have to start respiring anaerobically as well.
- In anaerobic respiration, the glucose is only partially broken down, and lactic acid is also produced. All animals that respire anaerobically produce lactic acid by the same process.
- The lactic acid builds up in the muscles, which gets painful and makes your muscles fatigued.
- The advantage is that at least you can keep on using your muscles.
- After resorting to anaerobic respiration, when you stop exercising you'll have an oxygen debt. Basically you need extra oxygen to break down all the lactic acid that's built up and to allow aerobic respiration to begin again. So you need to keep breathing hard for a while.
Lactic acid formula
glucose → lactic acid
C6H12O6 → 2C3H6O3
Plants and fungi produce ethanol and CO2
- Under certain conditions plants may also have to resort to anaerobic respiration, e.g. in waterlogged soil (where there is little or no oxygen) plant root cells respire anaerobically.
- Some fungi (such as yeast) can respire anaerobically too.
- Anaerobic respiration in plants and fungi produces ethanol and carbon dioxide instead of lactic acid. This is the equation:
glucose → ethanol + carbon dioxide
C6H12O6 → 2C2H5OH + 2C02
Aerobic respiration
Conditions - Oxygen present
Substrate - Glucose (or other organic molecules)
Products - Carbon dioxide and water
Energy transferred - Lots - 32 ATP made
Anaerobic respiration
Conditions - Not enough oxygen present, e.g. during vigorous exercise, in waterlogged soils
Substrate - Glucose (or another organic molecule)
Products -
- In animals - lact acid
- In plants and some fungi (e.g. yeast) - ethanol and carbon dioxide)
Energy transferred - much less - 2 ATP made
Biological Molecules can be broken down
Carbohydrates, proteins and lipids can be broken down so that energy can be transferred to ATP through respiration - the energy stored in ATP is then available for the cell to use.
Carbohydrates are made up of simple sugars
- Carbohydrate molecules contain the elements carbon, hydrogen and oxygen.
- The smallest units, monomers, are simple sugars e.g. glucose or fructose molecules.
- These can be joined in long chains, polymers, to make large, complex carbohydrates e.g. starch and glycogen.
- The polymer molecules can be broken down back into sugars again when the chemical bonds between the monomers are broken.
- In the body, carbohydrates are broken down (digested) by enzymes in the mouth and small intestine.
Proteins are made up of amino acids
- Proteins are polymers that are made up of long chains of monomers called amino acids.
- Amino acids all contain carbon, nitrogen, hydrogen and oxygen atoms.
- In the body, proteins are broken down by enzymes in the stomach and small intestine.
Lipids are made up of fatty acids and glycerol
- Lipids (fats and oils) are made from glycerol and three fatty acids.
- Unlike carbohydrates and proteins they are NOT polymers because they don't form a long chain of repeating units.
- Lipids contain carbon, hydrogen and oxygen atoms.
- In the body, lipids are broken down by enzymes in the small intestine.
Plants are able to make their own food
- During photosynthesis, photosynthesis organisms, such as green plants and algae, use energy from the Sun or an artificial source to make glucose.
- Some of the glucose is used to make larger, complex molecules that the plants or algae need to grow. These make up the organism's biomass - the mass of living material.
- The energy stored in the organisms' biomass then works its way through the food chain as animals eat them and each other. So ultimately, photosynthetic organisms support nearly all life on Earth.
- Photosynthesis happens inside chloroplasts - they contain chlorophyll which absorbs light. Energy is transferred to the chloroplasts from the environment by light.
- Photosynthesis is an endothermic reaction - energy is transferred from the environment during it.
- Photosynthesis actually happens in two main stages. First, energy is transferred by light is used to split water into oxygen gas and hydrogen ions.
- Carbon dioxide gas then combines with the hydrogen ions to make glucose.
Oxygen production- the rate of photosynthesis
- The rate of photosynthesis is affected by light intensity, concentration of carbon dioxide and temperature. Any of these three factors can become the limiting factor. This just means that it's stopping photosynthesis from happening any faster.
- You can investigate how each of the different factors affect the rate of photosynthesis. A classic way to do this is to use pondweed and to measure oxygen production.
- The rate at which the pondweed produces oxygen corresponds to the rate at which it's photosynthesising - the faster the rate of oxygen production, the faster the rate of photosynthesis.
Pondweed experiment
- The pondweed is left to photosynthesise for a set amount of time. As it photosynthesises, the oxygen released will collect in the capillary tube.
- At the end of the experiment, the syringe is used to draw the gas bubble in the tube up alongside a ruler and the length of the gas bubble is measured. This is proportional to the volume of oxygen produced.
- The experiment is then repeated to test a range of values for the factor being investigated, e.g. a range of different temperatures.
- Variables other than the one being investigated should be kept the same e.g. the other limiting factors, the time the pondweed is left for.
Not enough light slows down photosynthesis
- Light transfers the energy needed for photosynthesis.
- As the light level is raised, the rate of photosynthesis increases steadily - but only up to a certain point.
- Beyond that, it won't make a difference - it'll be either the temperature or the carbon dioxide level which is the limiting factor.
- In the lab you can investigate light intensity by moving a lamp closer to or further away from your plant.
- But if you just plot the rate of photosynthesis against "distance of lamp from the plant", you get a weird shaped graph. To get a proper graph you need to measure the light intensity at the plant using a light meter.
Light intenisty graph for photosynthesis
Too little carbon dioxide affects photosynthesis
- Carbon dioxide is one of the raw materials needed for photosynthesis.
- As with light intensity the concentration of carbon dioxide will only increase the rate of photosynthesis up to a point. After this the graph flattens out showing that carbon dioxide is no longer the limiting factor.
- As long as light and carbon dioxide are in plentiful supply then the factor limiting photosynthesis must be temperature.
- There are loads of different ways to control the concentration of carbon dioxide e.g. dissolve different amounts of sodium hydrogen-carbonate (which gives off carbon dioxide) in the water.
The rate of photosynthesis - Carbon dioxide
A graph showing the rate of carbon dioxide during photosynthesis:
The rate of photosynthesis - Light
- Usually, if the temperature is the limiting factor it's becasue it's too low - the enzymes needed for photosynthesis work more slowly at low temperature.
- But if the plant gets too hot, the enzymes it needs for photosynthesis and its other reactios will be denatured - the rate of reaction decreases dramatically.
- This can start to happen at about 45°C(pretty hot for outdoors, but greenhouses can get that hot if you're not careful).
- Experimentally, the best way to control the temperature of a boiling tube is to put it in a water bath.
The rate of photosynthesis - Light
A graph to show how light affects the rate of photosynthesis:
Related discussions on The Student Room
- Biology immunity question »
- Alevel bio synoptic essay »
- OCR A GCSE Biology Paper 3 Higher Tier [16th May 2023] Exam Chat »
- Viruses »
- My dad doesn’t wash his hands after pouring bleach »
- AQA GCSE Biology Paper 1 (Higher Tier) 2022 »
- weather »
- computer »
- BTEC science unit 12 assigment »
- Eduqas A-Level Biology Component 3 [21st June 2023] Exam Chat »
Comments
No comments have yet been made