Most elements contain a mixture of isotopes, each in a different amount (abundance) and each with a different mass.
Example:
A sample of bromine contains 53% bromine-79 and 47% bromine-81.
Relative atomic mass = ((53*79)+(47*81)/100 = 79.9
Relative atomic mass = (a*b)+(c*d) / 100 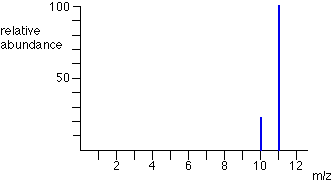
Comments
Report