AS BIOLOGY - EVERYTHING on biological molecules OCR F212
These cards will help with OCR Biology AS unit F212 biological molecules. Notes are in my own words and are based upon the CGP guide and OCR textbook as well as notes from my lessons.
- Created by: kyle
- Created on: 08-05-12 09:15
WATER - HYDROGEN BONDING
1 - Water is one atom of oxygen joined to two atoms of hydrogen by shared electrons.
2 - Becasue the shared negative hydrogen electrons are attracted to the oxygen atom, the other side of the hydrogen is left with a slight positive charge.
3 - The unshared negative electrons on the oxygen atom give it a slight negative charge.
4 - This makes the molecule of water polar ie. has a negative charge on one side and a positive charge on the other.
5 - The negatively charged oxygen atoms of water attract the positively charged hydrogen atoms of other water molecules.
6 - This attraction is known as a HYDROGEN BOND.
This hydrogen bond gives water many useful properties.
WATER'S PROPERTIES - PART 1
1 - It requires a lot of energy to break the hydrogen bonds in water, so water has a high latent heat of evaporation. This is useful for organisms because evaporation of water is an efficient cooling system eg. when an organism sweats.
2 - It has a high specific heat capacity, which means water requires a lot of energy to heat it up, meaning it stops rapid temperature changes for organisms, providing them with a stable temperature and stable environment.
3 - Transparency allows plants underwater to undergo photosynthesis.
4 - Ice is less dense than water, which provides a habitat for organisms eg. polar bear. The ice also insulates the water beneath so the aquatic life does not freeze.
5 - Surface tension provides habitat for organisms eg. pondskater
6 - Due to polarity, water is very cohesive. Useful in water transpiration.
WATER'S PROPERTIES - PART 2
7 - Water is an effective solvent. The negatively charged end will surround a positive ion (eg. sodium ion) whereas the positively charged end will surround a negative ion (eg. chloride ion). This means the ions will become dissolved. Eg. water can dilute toxic substances.
8 - Many organisms have a high internal percentage of water, so due to its high specific heat capacity, internal body temperature changes slowly. Allows enzymes to function correctly.
9 - It has a high density, allowing for floatation.
10 - Organsims can obtain oxygen from water.
PROTEINS - AMINO ACID STRUCTURE
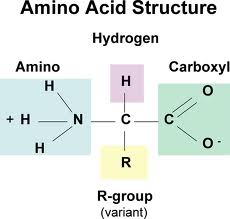
Amino acids contain the elements N, H, C and O. They can sometimes contain S and P. They are made up of an amine group, a variable side chain and carboxyl group.
PROTEINS - CONDENSATION REACTION
Amino acids form polypeptides when two or more bond together via a condensation reaction. They join when the H (from the amine group) of one amino acid, bonds together with the OH (from the carboxyl group) of another amino acid. This forms a peptide bond between the N and the C, and the H and OH combine to form water.
PROTEINS - HYDROLYSIS REACTION
Polypeptides can also be converted back into separate amino acids via a hydrolysis reaction. This is just the reverse of a condensation reaction.
PROTEINS - FOUR LEVELS OF STRUCTURE
PRIMARY - Simply the sequence of amino acids in a straight polypeptide chain.
SECONDARY - The polypeptide chain doesn't remain flat. Hydrogen bonds form between amino acids in the chain and make the chain coil into an alpha helix structure or beta pleated sheet.
TERTIARY - The secondary structure is folded further into a complex 3 dimensional shape. It is maintained by the formation of more bonds between different parts of the polypeptide chain. For proteins made of single polypeptide chains, this is their final 3D structure. (For types of bonds see next card)
QUATERNARY - Some proteins are made of several different polypeptide chains held together by bonds. For proteins made of more than one polypeptide chain, this is their final 3D structure.
NO IMAGES ARE INCLUDED BUT I RECOMMEND FINDING SOME.
PROTEINS - BONDING BETWEEN STRUCTURES
PRIMARY - Peptide bonds between amino acids.
SECONDARY - Hydrogen bonds between nearby amino acids.
TERTIARY - Ionic interactions are weak attractions between different negatively and positively charged parts of the molecule.
Disulphide bonds form between two sulfur atoms (which are on separate amino acids) when each molecule comes close together.
Hydrophobic and Hydrophilic interactions. When hydrophobic (water repelling) groups are close together in a protein, they tend to clump. This means the hydrophilic (water attracting) groups are pushed to the outside, which affects how the protein folds up into its final structure.
Hydrogen bonds as well
QUATERNARY - All the bonds mentioned above. Is determined by the tertiary structure.
GLOBULAR PROTEINS
Some proteins are globular eg. Haemoglobin. They are round and compact proteins made up of multiple polypeptide chains.
Haemoglobin is made up of 4 polypeptide chains (2 alpha helix and 2 beta pleated sheet) held together by disulphide bonds. The chains are coiled in such a way that the hydrophilic parts are on the outside of the molecule and hydrophobic parts are on the inside, thus making the protein soluble. This solubility is key for haemoglobin because it allows it to be easily transported in the blood.
Each chain in haemoglobin is associated with a prosthetic group called haem which contains Fe 2+. Oxygen can bind to the iron and so therefore, each haemoglobin molecule can combine with 4 oxygen molecules.
FIBROUS PROTEINS
Some proteins are fibrous eg. Collagen. They are 3 helical polypeptide chains (also known as a triple helix) that are tightly coiled round each other to form a rope shape.
The chains are held together by lots of bonds such as hydrogen bonds and disulphide bonds making them strong. However, they are insoluble.
Collagen fibrils can be formed when collagen molecules form covalent bonds between one another.
Due to their strength, they provide mechanical support to arteries, tendons, bones, cartilage and connective tissue. Minerals can also bind to the triple helix to increase its rigidity.
CARBOHYDRATES - GLUCOSE
Carbohydrates contain the general formula (CH2O)n. Monosaccharides are simple sugars with the previous general formula eg. Glucose (a hexose sugar meaning it contains 6 carbons). There are two types of glucose. ALPHA glucose and BETA glucose. The subtle difference is that the H and OH are reversed on beta glucose on carbon 1. They are structural isomers of each other. A way to remember the difference is --> Beta glucose has H on Bottom.
POLYSACCHARIDES
Polysaccharides are formed from the condensation reaction between three or more monosaccharides. Two monosaccharides form a disaccharide. The hydrogen from carbon 1 hydroxyl group binds with the OH on the carbon 4's hydroxyl group of an adjacent monosaccharide to form a 1-4 glycosidic bond and water. The reverse of this process is once again hydrolysis. Two alpha glucose molecules bonded together form maltose. Lots of alpha glucose molecules bonded together form amylose.
STARCH
Cells main source of energy is glucose. Excess glucose is stored as starch, which is broken down for energy when needed. Starch is made up of two polysaccharides (alpha glucose amylose and alpha glucose amylopectin).
Amylose is a long unbranched chained of alpha glucose molecules joined together by 1-4 glycosidic bonds. The molecules join at a slight angle which, after many repetitions, forms a spiral molecule/coiled structure with 6 glucose molecules per 1 turn. This makes it compact, making it good for storage as you can fit more in a smaller space.
Amylopectin is made up of alpha glucose joined together mainly by 1-4 glycosidic bonds. However, amylopectin also contain 1-6 glycosidic bond which creates a long, branched chain. This allows enzymes to reach the glycosidic bonds easily and allows glucose to be released quicker.
Starch is insoluble so does not affect the water potential of the cell. It is too large to move across membranes, so it is stored where it is made. Finally, it is made up of a large number of glucose molecules therefore, there is a large supply of respiratory substrate.
CELLULOSE
Cellulose is made of long, unbranched chains of beta glucose. The molecules are joined by beta 1-4 glycosidic bonds. Because they lie in straight chains, hydrogen bonds form between adjacent chains, forming strong fibres called microfibrils. As there are many hydrogen bonds, the microfibrils are firmly held together.
Microfibrils group together to form macrofibrils which are all parallel and orientated in the same direction. Then, in successive layers, the macrofibrils are orientated in different directions creating a mesh-like structure.
Macrofibrils are interwoven making cell walls rigid. The cell walls are fully permeable due to the channels between the different layers.
GLYCOGEN
Animal cells get energy from glucose. But the excess is stored as glycogen. Glycogen is a polysaccharide of alpha glucose molecules joined together mainly by 1-4 glycosidic bonds but with some 1-6 glycosidic bonds, causing the structure to be branched.
The glycogen is similar to that of amylopectin but has LOADS MORE branches. This is important because the enzyme that breaks down glycogen works from the terminal (end) glucose group. If there are loads of branches, there are lots of terminal groups so energy can be released a lot quicker, which is important for animals.
Glycogen is once again compact, insoluble and is a large molecule so can store lots of energy.
LIPIDS
Lipids are fats and oils. They are not polymers like proteins and carbohydrates. They are made up of two subunits called fatty acids and glycerol. They contain the elements C, H and O. The structural formula of glycerol and a fatty acid are below;
GLYCEROL FATTY ACID
The R group on the fatty acid is a long hydrocarbon tail. Different fatty acids have different hydrocarbon tails. If the tail contains only single carbon-carbon bonds then it is said to be saturated. If it only contains any double carbon=carbon bonds then it is said to be unsaturated.
TRIGLYCERIDES
Triglycerides are formed through a condensation reaction. The 3 hydroxyl groups of glycerol bond with the carboxyl group of 3 fatty acids to form ester bonds and 3x water.
Triglycerides are mainly used as energy storage molecules. The long hydrocarbon tails of the fatty acid contain lots of chemical energy. Lipids therefore contain twice as much energy per gram than carbohydrates. Also, the fact that they are insoluble prevents swelling of cells by osmosis. The triglycerides bundle together as insoluble droplets as the tails are hydrophobic, so they face inwards, whilst the glycerol heads face outwards.
PHOSPHOLIPIDS
Phospholipids are very similar to Triglycerides. Some lipids found in cell membranes are phospholipids. The difference in structure is that one of the fatty acid tails in a triglyceride is replaced by a phosphate group. The phosphate group is hydrophilic whilst the tails once again are hydrophobic. This helps create the phospholipid bilayer found in cell membranes.
CHOLESTEROL
Cholesterol is a type of lipid also found in cell membranes. It has hydrocarbon ring structure attached to a hydrocarbon tail. The hydrocarbon ring also has a polar hydroxyl group which makes cholesterol soluble in water. However, it is not soluble in blood so has to be transported by proteins known as lipoproteins.
hydroxyl group Hydrocarbon ring Hydrocarbon Tail
BIOCHEMICAL TESTS PART 1
STARCH - use a pipette to place a drop of starch on a spotting tile. Add a drop of iodine dissolved in potassium iodide solution. Result is positive if colour changes from browny-orange to blue-black.
PROTEIN - Put your solution into a test tube. The test solution needs to be alkaline so add a few drops of sodium hydroxide solution. Then add an equal volume of copper (II) sulphate solution. Result is positive if a lilac (mauve) solution/layer forms.
LIPIDS - Put your substance into a test tube. Add ethanol and shake thoroughly for a minute before pouring the solution into a test tube with cold water. Result is positive if a white emulsion is formed on the surface of the water.
REDUCING SUGAR - Place your substance into a test tube and add an excess of benedict's reagent and heat. Do not let it boil. Result is positive if it forms a coloured precipitate ranging from blue - green - yellow - orange - brick red depending on concentration.
BIOCHEMICAL TESTS PART 2
NON-REDUCING SUGAR - Carry out reducing sugar test to prove it isn't a reducing sugar. Then boil your solution with dilute hydrochloric acid and then neutralise it with sodium hydrogencarbonate. Then just carry out the benedict's reagent test again looking for the same colour changes.
COLORIMETRY (determine conc. of glucose) - Make several different but known concentrations of glucose, each having the same volume. Carry out a benedict's reagent test using same amount for each one. It must be in excess in order to react with all of largest known concentration of glucose. Remove precipitates formed using a funnel and use a colorimeter to measure the absorbance of the remaining solutions. Draw out a calibration curve and then your are able to work out concentration of unknown solutions by using the absorbance value.
The colorimeter works by measuring the absorbance of light by the solution. More concentrated solution will absorb more light. As more glucose results in a paler solution, then the higher the glucose concentration, the lower the absorbance of the solution.
QUICK OVERVIEW OF BIOLOGICAL MOLECULES
CARBOHYDRATES - (C,H,O) - component is monosaccharides - composed of monomers - form glycosidic bonds - condensation reaction to form them - hydrolysis to break them
LIPIDS - (C,H,O) - components are fatty acids and glycerol - NOT composed of monomers - form ester bods - condensation reaction to form them - hydrolysis to break them
PROTEIN - (C,H,O,N) - component is amino acid - composed of monomers - form peptide bonds - condensation reaction to form them - hydrolysis to break them
Related discussions on The Student Room
- I enjoy chem practicals more than bio practicals. Is a chem degree right for me? »
- Biochemistry vs Chemistry vs Natural Sciences »
- Biochemistry at University »
- Any good youtube channels for Bio + Chem a levels? »
- Access to Science course »
- A-level Biology Study Group 2023-2024 »
- Paper 3 AQA a Level biology »
- exams 2022 »
- AS/A Level Chemistry Study Group 2023/2024 »
- 25 mark essay question »
Comments
Report