The famous experiment performed by Geiger and Marsden, for Rutherford.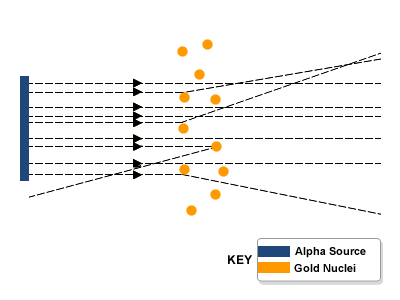
- Alpha particles were fired at a thin sheet of gold foil.
- About 99% of the alpha particles went straight through the foil and some were deflected at small angles. This means that most of the atom is empty space
- However, a very small number of alpha particles were scattered at angles greater than 90 degrees
- They concluded that this showed the existence of a tiny positive nucleus of size 10^(-15)m
Comments
No comments have yet been made