Allotrope - Different structural forms of the same element.
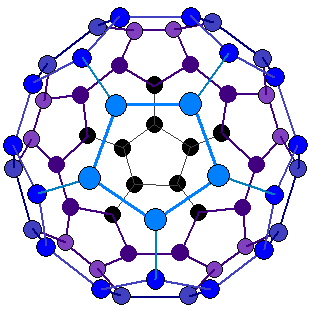 Fullerenes:
Fullerenes:
- Each Carbon atom is covalently bonded to 3 other carbon atoms.
- Buckminsterfullerene has a spherical structure and has 60 atoms.
- Weak intermolecular forces and so have low melting points and makes it soft and slippery.
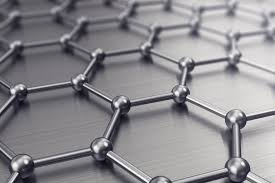 Graphene:
Graphene:
- Sheet of carbon atoms 1 atom thick.
- Light weight and strong.
- Alows free electrons to move across its surface and so is a good electrical conductor.
Comments
No comments have yet been made