Ionisation of weak acids – Higher tier
Strong acid, such as hydrochloric acid, ionise fully in water:
HCl(aq) → H+(aq) + Cl–(aq)
Their aqueous solutions have a high concentration of hydrogen ions, H+. This gives them a low pH.
Carboxylic acids are weak acids. They do not completely ionise when they are dissolved in water. Instead only some of their molecules ionise to form H+ ions:
CH3COOH(aq) 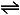 CH3COO–(aq) + H+(aq)
CH3COO–(aq) + H+(aq)
This means that an aqueous solution of a weak acid will have a higher pH compared to the same concentration of an aqueous solution of a strong acid
Comments
No comments have yet been made