Alcohols and Haloalkanes
- Created by: michaaaaaaaaaaar
- Created on: 02-04-18 10:17
nomenclature of alcohols
General formula: CnH2n+1OH.
Suffix -ol in place of the 'e' at the end of the name, eg ethane is ethanol.
If there are two -OH groups the molecule is a diol and if there is 3 it is an triol, eg ethane-1,2-diol.
Primary, Secondary and tertiary alcohols
Polarity of alcohols
Generally polar because electronegative hydroxyl group pulls the electrons in the C-OH bond away from the carbon.
Hydroxyl group is polar, delta negative charge on oxygen, delta positive charge on the hydrogen atom.
Partial positive charge on the hydrogen atom in hydroxyl group can attract the lone pairs on an oxygen from a neighbouring molecule and form hydrogen bonds

physical properties of alcohols
Low volatility and relatively high boiling point compared to the corresponding alkane, eg ethanol boils at 78 degrees celcius where as ethane boils at 89 degrees because of the hydrogen bonds between the -OH groups of eachother.
Alcohols are soluble in water due to the hydroxyl group in the alcohol which is able to form hydrogen bonds with water molecules. As the length of the hydrocarbon chain increases, the solubility in water decreases because most of the molecule is non-polar so theres less attraction for the polar water molecules.
Substitution reaction of alcohols
Alcohols react with compounds containing halides eg NaBr.
Hydroxyl group replaced by halide. Alcohol becomes haloalkane.
Acid catalyst is required, H2SO4.
elimination reaction of alcohols
Alcohol mixed with with an acid catalyst mixture is then heated and water is then eliminated from the alcohol.The water molecule eliminated is made up of the hydroxyl group and a H bonded to a C.
Sometimes an E/Z isomer can be formed.
Combustion of alcohols
C-C and C-H bonds are broken and products are CO2 and H2O.
Burning alcohols is a simple way to oxidise them. A better way of oxidising alcohols is by using Potassium dichromate (K2CR2O7/H2SO4).
The orange dichromate ion is reduced to a green chromium ion (III).
Oxidation of tertiary alcohols
Do not react with potassium dichromate(IV).Only way to oxidise is by burning them.
A way to test for tertiary alcohols is to look at the colour change as the dichromate will stay orange instead of turning into a green chromium(III) ion because tertiary alcohols cant be oxidised. 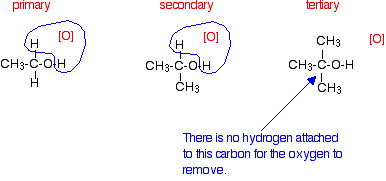
Reaction of primary alcohols
First oxidised to an aldehyde then carboxylic acid.
Heated with [O] and sulfuric acid in a test tube and an 'apple' smell is produced. To get just an aldehyde heat with a controlled amount of [O] in distillation apparatusso the aldehyde gets distilled off immediantely.
For a carboxylic acid to be formed it must be vigorously oxidised and heated under reflux which means the temperature can be increased and no volatile solvents, reactants or products are lost. Vaporised solvents are cooled, condensed and drip back into the reaction mixture.
Aldehyde is firstly made when heating alcohols under reflux but it stays in the reaction vessel with the oxidising agent and oxidises further into a carboxylic acid.
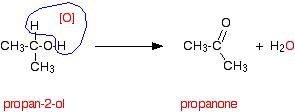
Oxidation of secondary alcohols
Ketone is produced.
Done under reflux.
Ketones cant be oxidised easily.
Reflux
The mixture is heated in a flask fitted with a Liebig condenser so the mixture can be continously boiled and the vapours evaporate into the condenser where they condense and are recycled back into the flask.
Heating is usually electrical which avoids naked flames that could ignite the compounds.
Distillation
Separates liquids with different boiling points by gently heating the mixture the substances evaporate out the mixture in order of increasing boiling point.Thermometer placed at neck of condenser to show the boiling point of the substance.

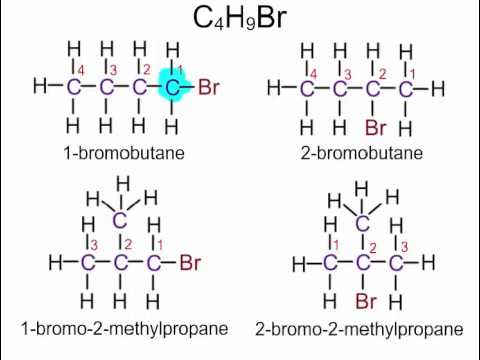
What are haloalkanes
An alkane with at least 1 halogen in place of a hydrogen atom.
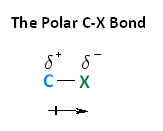
Polarity of haloalkanes
Halogens more electronegative than carbon so carbon-halogen bond is polar.
Carbon is electron deficient so can be attacked by a nucleophile- electron pair donor.
Nucleophilic substitution reactions
Nucleophile attacks a polar molecule, removes a functional group and puts itself in its place, general equation is: RX + Nu- -> RNu + X-
Carbon can only be bonded to four other molecules so the addition of the nucleophile breaks the bond between the carbon and the halogen. The pair of electrons are taken by the halogen and become a lone pair.
Hydrolysis of haloalkanes
Molecules are split by water molecules. Haloalkanes hydrolysed to make alcohols.
1) With a warm aqueous alkali, eg NaOH solution, in a nucleophillic substitution reaction. The nucleophile, in this case OH- kicks out the halogen and takes its place. General equation: RX + OH- -> ROH + X-
2) With water molecule. The reaction is slower but an alcohol still gets produced. General equation: RX + H20 -> ROH + H+ + X-
Bond enthalpy and hydrolysis
The rate of hydrolysis depends on the bond enthalpy, weaker C-X bonds break more easily, as you go down the halogens the rate of reaction increases.
You can use nucleophilic substitution of silver nitrate ions to compared reactivity of different haloalkanes. The time it takes for each silver halide to form a precipitate by sticking paper with a cross under the flask and time how long it takes until the cross can no longer be seen.
If the conditions are the same then iodoalkanes react the fastest to form a precipitate.
Identifying unknown haloalkanes
Put a sample of haloalkane in a test tube with water and silver nitrate and ethanol.
The colour formed tells you what halogen is present.
Yellow is iodoalkane.
Cream is a bromoalkane.
White is a chloroalkane.
CFCs
Haloalkanes that contain chlorine, flourine and carbon- with all the hydrogen atoms replaced.
They were used as refrigerants, propellants for aerosols, for generatingfoamed plastics like expanded polystyrene or polyurethane foam, and as solvents for dry cleaning and for general degreasing purposes. Until scientists found out that they were destroying the ozone layer.
CFCs and Ozone layer
Ozone layer is in the stratosphere and acts as a chemical sunscreen by absorbing a lot of UV radiation. Its formed naturally when an oxygen molecule is broken down into two free radicals by UV radiation.
The holes in the ozone layer are bad because they let UV radiation reach the earth. They're formed when CFCs travel into the upper atmosphere and absorb high-energy UV and split to form chlorine free radicals which catalyse the destruction of ozone. One Cl molecule can destroy 10,000 ozone molecules before it forms a stable compound because chlorine radicals are regenerated, so the overall reaction is: O3(g) + O(g) -> 2O2(g)
Reaction of Chlorine and Ozone
Nitrogen oxides and ozone layer
Nitrogen oxide (NO) radicals also destroy ozone.
They are produced by aircraft and car engines and in thunderstorms. They have the same affect on ozone as chlorine radicals
NO + O3 -> RO + O2
NO2 + O -> NO + O2
General equation of ozone being broken down, R stands for radical:
R + O3 -> RO + O2
RO + O -> R + O2
Because radicals act as catalyst the overall reaction is:
O3+ O -> 2O2
Alternatives to CFCs
HCFCs and HFCs are temporary being used until a better alternative is found.
CFCs stay in the atmosphere for a long time and cause a lot of damage, HCFCs are broken down much quicker than CFCs and still damage the ozone but have a smaller effect. HFCs are broken down and dont have chlorine so dont affect the ozone layer.HCFCs and HFCs are greenhouse gasses and contribute to global warming.
Most aerosols have been replaced by pump sprays or use nitrogen as the propellant and industrial fridges and freezers use ammonia as the coolant gas, and CO2 is used to expand polymers.However these substances still have negative effects but are less damaging then CFCs.
Ozone holes are still forming but are slowly shrinking.
Greenhouse gases
The main greenhouse gasses are water vapour, CO2 and methane. The C=O, O-H and C-H bonds abosrb IR radiation which make the bonds in the molecule vibrate more, the extra energy is passed onto other molecules in air by collisions which gives other molecules more energy and increases the temperature.
Not all greenhouse gasses contribute to the greenhouse affect, the contribution depends on:
- How much IR radiation one molecule of the gas absorbs
- How much of that gas is in the atmosphere
- How long the gas stays in the atomosphere for
Greenhouse affect is when various gases in the troposphere absorb some of the IR radiation the Earth re-admits, and re-admits it in all directions, including back to Earth.
Global warming and evidence
Human activities such as burning fossil fuels and food production such as cows and paddy fields(where rice is grown), and deforestation, have caused a rise in greenhouse gas concentration which enhances the greenhouse effect.
More heat is trapped and the Earth is getting warmer.
Scientists have collected evidence for if and why global warming and climate change is happening from analysis of air samples and seawater. The evidence shows that the Earths average temperature has increased dramatically over the past 50 years and that CO2 levels have also increased.
Although theres a correlation between CO2 and temperature does not mean that one thing causes the other.
Consequences and efforts to prevent global warming
Consequences:
Warmer oceans will expand, ice in polar regions would melt, sea levels would rise, more flooding. Because global warming means theres more heat energy in the system there could be stormier less predictable weather, less rain and droughts and crop failure which would lead to famine and increased number of refugees. Increased rainfall could lead to flooding and water diseases.
Prevention:
- Kyoto protocol- industrial companies promised to reduce their greenhouse gas emission to agreed levels, however the argeement came to an end in 2012.
- In the UK government created policies to use more renewable energy resources eg wind farms and solar power which reduces the need for coal. The government also encouraged the use of more energy-efficient alternatives to everday products such as energy saving light bulbs and electric cars and biofuel powered busses.
Related discussions on The Student Room
- Organic chemistry help »
- Organic chemistry questions »
- aqa a level chemistry paper 2 assessed topics »
- year 12 study journal! »
- Chemistry alevel mechanisms »
- Required practicals chemistry A level »
- Functional Groups Revision »
- Ask Us Anything! »
- Grade Growth Chronicles | From C's to A's (23-24) »
- A level chemistry »
Comments
No comments have yet been made